César Máximo Fuertes Ruitón
Martha Helena Jahuira-Arias
Original article
Preliminary phytochemical analysis and in vitro antifungal activity of the ethanolic extract of the leaves of Solanum hispidum pers. collected in the locality in Obraje – Peru
Jannelle Cyndi Mendoza–León ![]() 1,2, Master’s Degree in Plant Resources and Therapeutics
1,2, Master’s Degree in Plant Resources and Therapeutics
César Máximo Fuertes Ruitón ![]() 2, Doctor of Pharmacy and Biochemistry
2, Doctor of Pharmacy and Biochemistry
Martha Helena Jahuira-Arias ![]() 1,2, Doctor in Biological Sciences
1,2, Doctor in Biological Sciences
1 Instituto Nacional de Salud – Centro Nacional de Control de Calidad, Lima, Peru.
2 Universidad Nacional Mayor de San Marcos, Lima, Peru.
This study is part of the thesis: Mendoza-León J. Actividad antifúngica del extracto etanólico de las hojas de Solanum hispidum Pers y citotoxicidad en líneas celulares de cáncer humano [master’s thesis]. Lima: Faculty of Pharmacy and Biochemistry, Universidad Nacional Mayor de San Marcos; 2022.
ABSTRACT
Objective. To analyze and determine the in vitro antifungical activity of the ethanolic extract of the leaves of Solanum hispidum Pers. Materials and methods. We carried out a preliminary qualitative phytochemical analysis by color and precipitation reactions. We evaluated the in vitro antifungical activity against Candida albicans, Aspergillus brasilensis and Trichophyton mentagrophytes by using the agar well diffusion method and the minimum inhibitory concentration (MIC) assay. Results. Preliminary qualitative phytochemical analysis showed the presence of phenolic compounds, tannins, flavonoids, steroids, alkaloids and saponins. In vitro antifungal activity was demonstrated for all fungal cultures with inhibition halos between 23 to 26 mm. The MIC values were 125, 250, and 125 μg/mL for C. albicans, A. brasilensis, and T. mentagrophytes, respectively. Conclusions. The ethanolic extract of the leaves of Solanum hispidum Pers. contains important secondary metabolites and has moderate antifungical activity.
Keywords: Antifungical; in vitro; phytochemical; plant extracts (source: MeSH NLM).
INTRODUCTION
Plant extracts are widely used in the treatment of diseases, particularly as antifungals. Currently, research focused on their active biological components is promising, and the World Health Organization (WHO) has proposed that traditional medicine be considered for inclusion in the health care system (1).
Dermatomycosis is one of the most frequent skin diseases, and there are numerous epidemiological studies in our country on its incidence in the population (2). Drug resistance, therapeutic failures, adverse effects, and toxicity regarding the use of conventional antifungal drugs represent a problem, so it is necessary to seek new alternatives for treatment (3). Traditional medicine is an important option; however, it needs to be scientifically validated towards conventional medicine.
The Solanaceae family is one of the most diverse and the genus Solanum is widely distributed in Peru; Solanum hispidum Pers is found between 2500 to 3500 m altitude (4). This plant grows abundantly in Carhuaz, where it is known as ñahui pashta and is traditionally used by the local population to treat foot mycosis by topical application of the fruit contents (5).
Previous studies have demonstrated the in vitro antifungal activity of several species of the genus Solanum, such as Solanum crysotrichum against pathogens such as Trichophyton mentagrophytes, Trichophyton rubrum and Trichophyton gypseum (6). Subsequently, clinical studies were conducted on a topical solution derived from the methanolic extract of its leaves, which showed effectiveness against Tinea pedis (7). Solanum melongena showed antifungal activity against Trichophyton mentagrophytes, Trichophyton rubrum, Trichopyton tonsurans, Candida albicans and Trichosporon beigeii (8). In addition, it has been reported that Solanum xanthocarpum inhibits the growth of Aspergillus fumigatus, Aspergillus flavus and Aspergillus niger (9); other recent studies also demonstrated antifungal activity against Candida albicans (10). The species Solanum nigrum L has antifungal activity against Trichophyton rubrum, Trichophyton tonsurans, Trichophyton mentagrophytes, Microsporum gypseum and Candida albicans (11); alkaloids, flavonoids, coumarins, tannins and saponins were found among its phytochemical compounds (12).
The leaves of Solanum hispidum Pers are used as an antifungal in Mexican folk medicine, and its antifungal activity against Trichophyton mentagrophytes, Trichophyton rubrum, Aspergillus niger and Candida albicans has been demonstrated; the strains that showed greater sensitivity were Trichophyton mentagrophytes and Trichophyton rubrum; in addition, steroidal saponins were identified and isolated (13). On the other hand, the recent study by Retamozo (14) reported the abundance of steroidal glycoalkaloids as main secondary metabolites in leaves and fruits of this species. However, there is no study in Peru on the evaluation of their properties against fungal agents. Therefore, this study aims at the preliminary phytochemical analysis and antifungal activity of the ethanolic extract of leaves of Solanum hispidum Pers.
|
KEY MESSAGES |
|
Motivation for the study: this study seeks to validate the ethnobotanical use of Solanum hispidum Pers as an antifungal, as well as to evaluate its phytochemistry in order to determine the main metabolites and demonstrate its in vitro activity against different fungal agents. Main findings: we mainly found steroids and alkaloids in the extract, as well as moderate antifungal activity against C. albicans ATCC 10231, A. brasilensis ATCC 16404 and T. mentagrophytes ATCC 9533. Implications: it is necessary to continue researching this topic, with the purpose of obtaining topical and affordable phytopharmaceuticals with antifungal activity. |
MATERIALS AND METHODS
Collection of plant material
Fresh leaves of Solanum hispidum Pers were randomly collected from different specimens distributed in the department of Ancash, province of Carhuaz, district of Acopampa, locality of Obraje, an altitude of 2750 m (Figure 1).
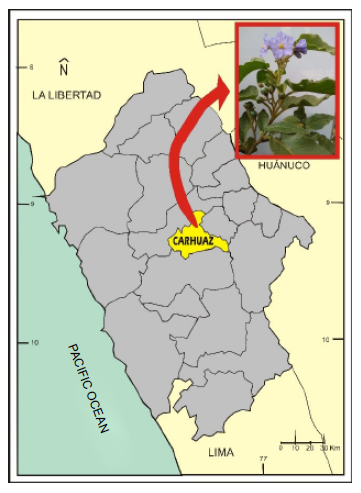
Figure 1. Location where the leaves of Solanum hispidum Pers were collected.
The species was taxonomically identified and certified by the San Marcos herbarium of the Natural History Museum of the Universidad Nacional Mayor de San Marcos (code: 053-USM-2017).
Obtaining the ethanolic extract of the plant
The leaves of Solanum hispidum Pers were washed with distilled water and initially dried at room temperature for seven days, the drying process was then completed at 40 °C in an oven with circulating air for five days; subsequently, the leaves were crushed and ground until a uniform fine powder was obtained (15).
The powder was mixed with 90% ethanol in a 1:10 ratio in an amber glass bottle which was kept at room temperature for seven days with frequent manual shaking. Then, the extract was filtered using gauze and 20 µm cellulose filter paper; subsequently, the solvent was evaporated under reduced pressure in a rotary evaporator® (Buchi-R-100) at 40 °C and 60 rpm (15,16). The dried extract was stored refrigerated at 2 to 8 °C until use.
Solubility test
To 20 mg of the stabilized ethanolic extract of Solanum hispidum Pers we added 1 mL of each of the following solvents: distilled water, ethanol, methanol, ethyl acetate, chloroform, diethyl ether and n-hexane. Then each tube was shaken and the results were observed for a maximum of 10 min (17).
Phytochemical analysis
To determine the presence or absence of the main secondary metabolites, we carried out qualitative staining and precipitation tests using the standard chemical methods described by Lock (18).
Microorganisms
We used strains of Candida albicans ATCC 10231, Trichophyton mentagrophytes ATCC 9533 and Aspergillus brasilensis ATCC 16404 provided by the Cepario of the Microbiology and Biological Laboratory of the Instituto Nacional de Salud.
T. mentagrophytes and A. brasilensis strains were grown on Sabouraud dextrose agar (ADS) for 7 and 10 days, respectively. C. albicans was incubated in Sabouraud dextrose broth for 48 h, the incubation temperature was 20 to 25 °C. The strains were suspended and adjusted with a spectrophotometer to a concentration of 1 x 106 CFU/mL for C. albicans and 1 x 105 CFU/mL for T. mentagrophytes and A. brasiliensis, respectively (19).
In vitro antifungal activity
The antifungal activity of the ethanolic extract of Solanum hispidum Pers leaves was demonstrated using the agar well diffusion method (19).
We inoculated 1 mL of the fungal suspension (0.5 x 105 CFU/mL for C. albicans and 0.5 x 104 CFU/mL for T. mentagrophytes and A. brasiliensis) into 20 mL of Sabouraud dextrose agar (ADS). It was mixed uniformly and poured homogeneously into Petri dishes, then when the surface solidified, 11 mm diameter wells were punched with a sterile stainless-steel punch; 100 µL of the ethanolic extract (25 mg/mL) was added to each well (19). Subsequently, the plates were incubated at 37 °C for 24 h for C. albicans, 72 h for A. brasiliensis, and seven days for T. mentagrophytes; dimethyl sulfoxide (DMSO) and distilled water were used as negative controls (20,21).
We evaluated the antifungal activity after the incubation time was finished by measuring the diameter of the inhibition zone in mm. The antifungal activity of the extract was evaluated by comparing the inhibition zones with standard antifungals for each microorganism (nystatin at 0.2 mg/mL ketoconazole at 0.2 mg/mL and fluconazole at 0.2 mg/mL) (19). Eight replicates were carried out for each strain.
Determination of the minimum inhibitory concentration (MIC)
For the determination of the minimum inhibitory concentration (MIC), we used the colorimetric microdilution method in microplate following the Clinical and Laboratory Standards Institute (CLSI) protocols (22,23) modified by Liu (24) and Fernandez (20).
We obtained suspensions in RPMI 1640 (Sigma-Aldrich) with resazurin for each strain: ranges of 0.5 - 2.5 x 103 CFU/mL for C. albicans and ranges of 0.6 to 3 x 104 CFU/mL for A. brasiliensis and T. mentagrophytes, respectively. In addition, serial dilutions of ethanolic extract of Solanum hispidum Pers leaves were prepared in RPMI 1640 medium (sigma-Aldrich) with resazurin, the evaluated concentrations ranged from 3.91 to 2000 µg/mL. Each assay was carried out in triplicate for each strain. Plates were incubated aerobically at 37 °C for 24 h for C. albicans, five days for A. brasiliensis and seven days for T. mentagrophytes. We visually evaluated the results after the incubation period was over; when the biological activity was inhibited the original color decreased noticeably (24).
In all assays, the antifungals ketoconazole and fluconazole in RPMI medium (22,23) with resazurin 0.05 mg/mL and sterility controls containing the culture medium with resazurin 0.05 mg/mL without the microorganism were used as positive controls (21).
For the interpretation of antifungal activity, we used the qualitative criteria described by Holets et al. (2002) (25), i.e., MIC < 100 µg/ML (good), 100 to < 500 µg/mL (moderate), 500 to 1000 µg/mL (weak).
Statistical analysis
The data obtained were analyzed using MINITAB 19 software. We carried out the descriptive and statistical analysis of the variables.
Ethical aspects
This project was approved by the Institutional Research Ethics Committee of the Instituto Nacional de Salud (CIEI -INS), RD No. 533-2019 OGITT/INS. We used strains from the (ATCC) maintained at the Microbiology and Biological Laboratory of the Instituto Nacional de Salud at -70 ºC. No patients were involved in this study.
RESULTS
Solubility test
The solubility tests results are described in Table 1, which shows that the stabilized ethanolic extract of Solanum hispidum Pers leaves was poorly soluble (+) in the solvent n-hexane; soluble (++) in distilled water, ethyl acetate, chloroform, diethyl ether; and very soluble (+++) in alcoholic solvents such as ethanol and methanol. The decrease in solubility was directly proportional to the polarity index of the test solvent.
Table 1.Solubility of the stabilized ethanolic extract of Solanum hispidum Pers.
|
Solvent |
Polarity index |
Result |
|
Distilled water |
9.0 |
++ |
|
Ethanol |
5.2 |
+++ |
|
Methanol |
5.1 |
+++ |
|
Ethyl acetate |
4.4 |
++ |
|
Chloroform |
4.1 |
++ |
|
Diethyl ether |
2.8 |
++ |
|
n-hexane |
0.0 |
+ |
-: insoluble; +: slightly soluble; ++: soluble; +++: very soluble
Preliminary phytochemical analysis
The ethanolic extract obtained from leaves of Solanum hispidum Pers showed a variety of secondary metabolites; we identified phenolic compounds, tannins, flavonoids, steroids, alkaloids and saponins (Table 2).
Table 2. Secondary metabolites identified in the ethanolic extract of Solanum hispidum Pers.
|
Metabolite |
Test |
Result |
|
Phenolic compounds |
Ferric trichloride |
+ |
|
Tannins |
Gelatin - NaCl |
+ |
|
Flavonoids |
Shinoda |
+ |
|
|
Pews |
+ |
|
|
Sodium hydroxide |
+ |
|
Steroids |
Trichloroacetic Acid |
+ |
|
|
Liebermann-Burchard |
+ |
|
|
Rosenthaler |
+ |
|
|
Salkowski |
+ |
|
Alkaloids |
Draggendorf |
+ |
|
|
Mayer |
+ |
|
|
Bouchardat |
+ |
|
|
Sonneschein |
+ |
|
Saponins |
Foam |
+ |
|
Coumarins |
Hidroxilamina |
- |
|
Naphthoquinones |
Bornträger |
- |
|
Anthraquinones |
Bornträger |
- |
|
Cardiotonic glycosides |
Kedde |
- |
|
Sesquiterpene lactones |
Bajlet |
- |
|
Leucoanthocyanins |
Rosenheim |
- |
- : absence of the metabolite, +: presence of the metabolite
Coumarins, naphthoquinones, anthraquinones, cardiotonic glycosides, sesquiterpene lactones and leucoanthocyanins were not found in the ethanolic extract.
In vitro antifungal activity
In vitro antifungal activity was evaluated by the agar culture diffusion method against Candida albicans ATCC 10231, Aspergillus brasiliensis ATCC 16404 and Trichophyton mentagrophytes ATCC 9533; the activity was demonstrated by inhibition halos (Figure 2).
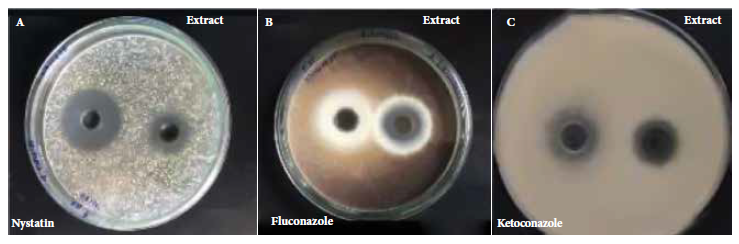
Figure 2. Representative experiment showing the comparison of the inhibition halos of each microorganism: A) Candida albicans ATCC 10231, B) Aspergillus brasiliensis ATCC 16404 and C) Trichophyton mentagrophytes ATCC 9533 against ethanolic extract of Solanum hispidum Pers leaves and standard antifungals (positive controls).
Table 3 presents the results of the antifungal activity of the leaves of Solanum hispidum Pers. The assay showed that the inhibition halo was 26 mm (± 0.38) for C. albicans, 23 mm (± 0.53) for A. brasiliensis and 25 mm (± 1.31) for T. mentagrophytes. The ethanolic extract of Solanum hispidum Pers leaves showed greater activity against C. albicans, however, its positive control (nystatin) presented a larger halo (30 mm) compared to the ethanolic extract.
Table 3. Antifungal activity of the ethanolic extract of Solanum hispidum Pers leaves against microorganisms.
|
Microorganism |
Ethanolic extract (mg/mL) |
Inhibition halo diameter |
|
Candida albicans |
25
mg/mL |
26 ±
0.38 |
|
Aspergillus brasiliensis |
25
mg/mL |
23 ±
0.53 |
|
Trichophyton mentagrophytes |
25
mg/mL |
25 ± 1.31 |
SD: standard deviation
Determination of the minimum inhibitory concentration (MIC)
Following the demonstration of antifungal activity, we evaluated the MIC. The results showed that the lowest concentration of the ethanolic extract of Solanum hispidum Pers leaves that completely inhibits growth of C. albicans was 125 µg/mL; for A. brasiliensis it was 250 µg/mL and for T. mentagrophytes it was 125 µg/mL; based on the criteria of antifungal activity, all of them showed moderate activity (Table 4).
Table 4. Minimum inhibitory concentration (MIC) values (µg/mL) of the ethanolic extract of Solanum hispidum Pers leaves against microorganisms.
|
Microorganism |
Minimum inhibitory concentration |
Antifungal activity criteria |
|
Candida albicans |
125 |
Moderate |
|
Aspergillus brasiliensis |
250 |
Moderate |
|
Trichophyton |
125 |
Moderate |
DISCUSSION
This study determined that the stabilized ethanolic extract of Solanum hispidum Pers leaves presents a higher solubility against ethanol and methanol, that is, with tendency to polar solvents, both of which are widely used; however, in this study we continued with ethanol due to its availability, considering, in addition, that most metabolites with antifungal activity have intermediate polarity and can be easily concentrated in this type of solvents (26). Our results agree with other studies carried out on crude extracts (24).
Qualitative analyses were carried out in order to detect the metabolites present in the ethanolic extract of Solanum hispidum Pers leaves. Our results showed the presence of multiple metabolites such as phenolic compounds, tannins, flavonoids, steroids, alkaloids and saponins; in addition, to verify their presence, we used four different tests for alkaloids and four differential tests for steroids with respect to triterpenoids (18), thus demonstrating the reliability of the results.
In this sense, the genus Solanum has been reported to have an abundance of alkaloids and steroids, such is the case of Solanum chrysotrichum (6,7), Solanum xanthocarpum (27), Solanum nigrum (11), Solanum surattense (28) and Solanum quitoense (29). Retamozo (14) was able to identify steroidal glycoalkaloids through qualitative quantitative tests in the same species, Solanum hispidum Pers; in addition, he analyzed the content of leaves and fruits, demonstrating a higher content in the fruit compared to the leaves; this also explained that the variability of the content is influenced by different factors such as vegetative state, time of collection, origin, etc. This confirms the presence of alkaloids and steroids as components of Solanum hispidum Pers. leaves extract.
We used a concentration of 25 mg/mL of the extract in DMSO, based on previous screening studies of ethanolic extracts of Peruvian plants with antifungal activity; Rojas et al. (19) and Quiroz (21) obtained favorable results using this concentration in 24 and 8 medicinal plants, respectively.
Regarding antifungal activity, the ethanol extract of Solanum hispidum Pers leaves showed an inhibitory effect on the growth of C. albicans, A. brasiliensis and T. mentagrophytes with inhibition zones between 23 and 26 mm. In this sense, Rojas et al. (19) mentioned that antifungal activity with inhibition halos greater than 18 mm, using the agar well diffusion method, is an indicator of good performance as a potential therapeutic agent.
The research carried out by Das et al. (8) demonstrated halos of 18 mm against the pathogen C. albicans using Solanum melongena; on the other hand, Shubha et al. (27) reported halos of 12 mm using Solanum xanthocarpum extract; both species belong to the Solanaceae family. In previous studies, Solanum nigrum and Solanum xanthocarpum species also showed activity against C. albicans by the disk diffusion method (26). In addition, a screening study of Peruvian plants revealed that a species of the Solanaceae family showed higher activity, evidencing inhibition zones of 19 mm with the agar well diffusion method (19). The results of this research show higher inhibition halos (≥ 23 mm) compared to studies against other species of the genus Solanum (8,19,26,27).
Also, our study showed moderate antifungal activity for C. albicans with values of 125 µg/mL, which are lower than the 256 µg/mL reported for Solanum mammosum species (30). Antifungal activity has also been reported against other fungal species such as Aspergillus sp or Solanum xanthocarpum against A. niger (10), showing antifungal activity with MIC of 250 µg/mL, our study also found this same value (250 µg/mL) against A. brasiliensis. For Trichophyton mentagrophytes we found a MIC of 125 µg/mL; similar results have been reported with Solanum mammosum with values of 256 µg/mL (30) for T. mentagrofphytes.
Many species of the Solanaceae family such as Solanum chrysotrichum (6,7), Solanum melogena (8), Solanum nigrum (11), Solanum xanthocarpum (23) and Solanum mammosum (30) also showed antifungal activity, which may be due to the presence of saponins, alkaloids, steroids and/or flavonoids, which may act individually or synergistically by a mechanism of action that remains unknown.
Both steroids and alkaloids have high biological activity and are a group of cyclic compounds that have been studied due to their antimicrobial effects, which has been confirmed through the isolation of bioactive compounds with potent in vitro antifungal activity (30).
One of the limitations of our study is that we used only the leaves and not the fruits, which are also used in the surrounding areas where the specimens were collected. This is due to the fact that they are not renewable and it was preferred not to affect their natural and habitual growth; nevertheless, we propose that the next stage, in order to complement this article, is to study the fractionation and characterization of the bioactive compounds of these fruits.
This in vitro study presents a preliminary phytochemical analysis of Solanum hispidum Pers. extract, in which it was possible to identify the main secondary metabolites, information not previously known, and to demonstrate the moderate in vitro antifungal activity of the ethanolic extract of Solanum hispidum Pers. leaves.
Acknowledgments: The authors would like to thank the support staff of the National Center for Quality Control of the Instituto Nacional de Salud, especially the research coordinator Roberto Torres Olivera for facilitating the use of the laboratories for the development of this research. To Jany Arias Tuco for the design and edition of the reference collection map.
Author contributions: JML participated in the conception, design, sample collection, data analysis and interpretation, and writing of the article. CFR participated in the design, data analysis and critical revision of the article. MJA participated in the design, writing, data analysis, and critical revision of the article. All authors reviewed, approved, and take responsibility for the final version of the manuscript.
Conflicts of interest: the authors deny any conflict of interest regarding this study.
Funding: the study was funded by the authors.
REFERENCES
1. Organización Mundial de la Salud. Estrategia de la OMS sobre medicina tradicional 2014-2023 [Internet]. Ginebra, Suiza; 2013 [cited 2022 Feb 10]. Available from: https://apps.who.int/iris/handle/10665/95008.
2. Bejar V, Villanueva F, Guevara JM, González S, Vergaray G, Abanto E, et al. Epidemiología de las dermatomicosis en 30 años de estudio en el Instituto de Medicina Tropical Daniel A. Carrión. Universidad Nacional Mayor de San Marcos. An Fac med. [Internet]. 2014 [cited 2019 Jul 1]; 75(2):167-72. Available from: http://www.scielo.org.pe/scielo.org.pe/scielo.php?script=sci_arttext&pid=S1025-55832014000200013&lng=es.
3. Sandoval N, Arenas R, Giusiano G, Garcia D, Chávez L, Zuñiga P. Diagnóstico y tratamiento de dermatofitosis y pitiriasis versicolor. Rev Med Hondur. [Internet]. 2012 [cited 2021 Aug 5]; 80(2): 66-74. Available from: www.bvs.hn/RMH/pdf/2012/pdf/Vol80-2-2012-8.pdf.
4. Särkinen T, Baden M, Gonzáles P, Cueva M, Giacomin L, Spooner D, et al. Listado anotado de Solanum L. (Solanaceae) en el Perú. Rev Peru Biol. 2015; 22 (1): 003-62. doi: 10.15381/rpb.v22i1.11121.
5. Mendoza León JC. Actividad antifúngica del extracto etanólico de las hojas de Solanum hispidum Pers y citotoxicidad en líneas celulares de cáncer humano. [Master’s thesis] Lima: Facultad de Farmacia y Bioquímica UNMSM; 2022.
6. Zamilpa A, Tortoriello J, Navarro V, Delgado G & Alvarez L. Five new steroidal saponins from Solanum chrysotrichum leaves and their antimycotic activity. J Nat Prod. 2002; 65(12):1815-9. doi:10.2021/np020261h.
7. Herrera-Arellano A, Rodríguez-Soberanes A, de los Angeles Martínez-Rivera M, Martínez-Cruz E, Zamilpa A, Alvarez L, et al. Effectiveness and tolerability of a standardized phytodrug derived from Solanum chrysotrichum on Tinea pedis: a controlled and randomized clinical trial. Planta Med. 2003 May; 69(5):390-5. doi: 10.1055/s-2003-39710.
8. Das J, Lahan JP, Srivastava RB. Solanum melongena: A potential source of antifungal agent. Indian J Microbiol. 2010; 50(1):62-69. doi:10.1007/s12088-010-0004-2.
9. Dabur R, Singh H, Chhillar AK, Ali M, Sharma GL. Antifungal potential of Indian medicinal plants. Fitoterapia. 2004 Jun; 75(3-4):389-91. doi: 10.1016/j.fitote.2004.01.015.
10. Garhewall S, Shiv G and Wast N. Anti-fungal activity fo Solanum xanthocarpum (kantkari) eaf extract. World Journal of Zoology. 2014; 9(2):111-4. doi: 10.5829/idosi.wjz.2014.9.2.83310.
11. Shivakumar SP & Vidyasagar GM. Antimycotic activity of low polar petroleum ether and interpolar methanolic young leaf extracts of Solanum nigrum L. Int Lett Nat Sci. 2015; 4 (1): 47-56. doi: 10.18052/www.scipress.com/ILNS.31.47.
12. Chang HL, García-Lopez A, Rosabal CY, Espinosa RA, Ramos EM, Remon RH. Caracterización fitoquímica y la evaluación de la actividad antibacteriana in vitro de los extractos de hojas y tallos de Solanum nigrum L. que crece en Cuba. Rev Mex Cienc. Farm [Internet]. 2013 [cited 2022 May 1] ; 44( 4 ): 30-35. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-01952013000400004&lng=es.
13. González M, Zamilpa A, Marquina S, Navarro V, Alvarez L. Antimycotic spirostanol saponins from Solanum hispidum leaves and their structure-activity relationship, J Nat Prod. 2004 Jun; 67(6): 938-41. doi: 10.1021/np0305019.PMID: 15217270.
14. Retamozo Montes A. Contenido de glicoalcaloides esteroidales totales en las hojas y frutos de Solanum hispidum Pers y Solanum radicans L y determinación de su bioactividad frente a Artemia salina. [Tesis de grado]. Ayacucho: Facultad de Ciencias de la Salud UNSCH; 2018.
15. Seidel V. Una introducción al aislamiento de productos naturales. En: Sarker, S., Nahar, L. (eds) Aislamiento de productos naturales. Métodos en Biología Molecular [Internet]. Humana Press vol 864; 2012 [cited 2021 Mar 5]. Available from: 10.1007/978-1-61779-624-1_2.
16. Singh J. Chapter 3 Maceration, Percolation and Infusion Techniques or the Extraction of Medicinal and Aromatic Plants. En: Handa S, Khanuja S, Longo G, Rakesh D. Extraction Technologies for Medicinal and Aromatic Plants [Internet]. Trieste: ICS UNIDO; 2008 [cited 2021 Jul 30]. Available from: https://www.unido.org/sites/default/files/2009-2010/Extraction.
17. Dominguez X. Métodos de investigación fitoquímica. Limusa; 1973.
18. Lock O. INVESTIGACIÓN FITOQUÍMICA. Métodos en el estudio de productos naturales. 3ª ed. Lima: PUCP; 2016.
19. Rojas R, Bustamante B, Bauer J, Fernandez I, Alban J, Lock O. Antimicrobial activity of select Peruvian Medicinal Plants. J Ethnopharmacol. 2003; 88(2-3): 199-204. doi: 10.1016/s0378-8741(03)00212-5.
20. Fernández TB, Cabañes F, Carrillo A, Esteban A, Inza I, Abarca L & Guarro J. Collaborative evaluation of optimal antifungal susceptibility testing conditions for dermatophytes. J Clin Microb.2002; 40(11): 3999-4003. doi: 10.1128/JCM.40.11.3999-4003.2022.
21. Ruiz Quiroz JR. Actividad antifúngica in vitro y concentración mínima inhibitoria mediante microdilución de ocho plantas medicinales. [Master’s thesis] Lima: Facultad de Farmacia y Bioquímica UNMSM; 2013.
22. Clinical Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeast; approved standard NCCLS document M27-A2. Wayne PA: National Committee for Clinical Laboratory Standard Institute. 2002.
23. Clinical Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard CLSI document M38-A2. Wayne PA: Clinical Laboratory Standards Institute; 2008.
24. Liu M, Seidel V, Katerere D & Gray A. Colorimetric broth microdilution method for the antifungal screening of plant extracts against yeast. Methods. 2007; 42: 325-329. doi: 10.1016/j.ymeth.2007.02.013.
25. Holetz F, Pessini G, Sanches N, Garcia D, Nakamura C, Filho B. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz. 2002; 97(7): 1027-1031. doi: 10.1590/S0074-02762002000700017.
26. Abbas K, Hussain T, Speed M, Javaid Z, Idrees A, Rasool S. Antimicrobial activity of fruits of Solanum nigrum and Solanum xanthocarpum. Acta Pol Pharm [Internet].2014 [cited 2022 Mar 3]; 71(3):415-21. Available from: https://www.ptfarm.pl/File/Acta_poloniae/2014/3/415.pdf.
27. Shubha KS, Sumana K, Lakshmidevi L. Antifungal Activity of Solanum xantocarpum Sch and Wend and Picrorhiza kurroa Royle ex Benth against Some Clinical Dermatophytes. Int J Curr Microbiol Appl Sci. 2016; 5(2): 236–44. doi: 10.2546/ijcmas.2016.502.026
28. Shivakumar T, Pasupuleti S, Kumar K, Rani S, Basaiahgari P, Pabbaraju N. Phytochemical and pharmacological activities of Solanum surattense Burm f - A review. J Appl Pharm Sci. 2017; 9(3):126-36. doi: 10.7324/JAPS.2019.90318.
29. Flechas H, Sanchez L, Silva J. Phytochemical screening and performance calculation of steroidal saponins from three provenances of Solanum quitoense Var. Septentrionale "naranjillo". Colombia for. [Internet]. 2008 [cited 2021 Aug 30]; 11(1): 201-11. Available from: http://scielo.org.co/php?Script=sci_arttext&pid=S0120-073920080001000013&ing=en.
30. Cabanillas B, Chassagne F, Vásquez P, Tahrioui A, Chevalier S, Vansteelandt M, et al. Pharmacological validation of Solanum mammosum L. as an anti-infective agent: Role of solamargine. J Ethnopharmacol. 2021;280(114473):1-8. doi: 10.1016/j.jep.2021.114473.
Correspondence: Martha Helena Jahuira Arias; jahuirah@gmail.com
Cite as: Mendoza -León JC, Fuertes Ruitón CM, Jahuira-Arias MH. Preliminary phytochemical analysis and in vitro antifungical activity of the ethanolic extract of the leaves of Solanum hispidum pers. collected in the locality of Obraje – Peru. 2022;39(3):321-7. doi: https://doi.org/10.17843/rpmesp.2022.393.11381.
Received: 24/05/2022
Approved: 07/09/2022
Online: 30/09/2022