Liz Romero
Oswaldo Tipiani
Steev Loyola
Jesús Tamariz
Original article
In vitro antimicrobial activity of bixa orellana l. Leaves extract against anaerobic bacteria associated to bacterial vaginosis and lactobacillusspp
Jenny Marcas ![]() 1, Biologist
1, Biologist
Liz Romero ![]() 1, Biologist
1, Biologist
Oswaldo Tipiani ![]() 2, Obstetrician gynecologist
2, Obstetrician gynecologist
Steev Loyola ![]() 1,3, Medical technologist, Master in Epidemiology
1,3, Medical technologist, Master in Epidemiology
Jesús Tamariz ![]() 1,3, Biologist, Doctor in Biological Sciences
1,3, Biologist, Doctor in Biological Sciences
1 Antimicrobial Resistance and Immunopathology Laboratory, Universidad Peruana Cayetano Heredia, Lima, Peru
2 Hospital Municipal de Surco, Lima, Peru
3 Medical school, Universidad Peruana Cayetano Heredia, Lima, Peru
ABSTRACT
Objective. To describe the in vitro antimicrobial activity of the methanolic extract of Bixa orellana L. leaves against anaerobic bacteria associated to bacterial vaginosis and Lactobacillus spp. Materials and methods. Eight ATCC reference strains; Gardnerella vaginalis, Prevotella bivia, Peptococcus niger, Peptostreptococcus anaerobius, Mobiluncus curtisii, Atopobium vaginae, Veillonella parvula, and Lactobacillus crispatus, and twenty-two clinical isolates; eleven Gardnerella vaginalis and eleven Lactobacillus strains, were included in the study. The antimicrobial susceptibility was determined by the agar diffusion method. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined by using agar dilution and a modified dilution plating method, respectively. Results. All ATCC reference strains showed high levels of susceptibility to the extract, except P. vibia, V. parvula and L. crispatus. Interestingly, all G. vaginalis clinical isolates and the G. vaginalis ATTC strain were the most susceptible to the extract, given their low MIC (1.0 – 2.0 mg/mL) and MBC (1.0 – 4.0 mg/mL) values, whereas, the Lactobacillus spp. clinical isolates and the L. crispatus ATCC strain were the least susceptible bacteria given their high MIC (32.0 mg/mL) and MBC (≥ 32.0 mg/mL) values. Conclusions. In vitro experiments suggest that the extract possesses selective antimicrobial properties given its high activity against bacterial vaginosis-associated anaerobic bacteria and low activity against Lactobacillus species.
Keywords: Bixa orellana; Vaginosis, bacterial; Plant Extracts; in vitro Techniques; Gardnerella vaginalis; Lactobacillus (Source: MeSH).
INTRODUCTION
Vaginal infections cause more than 10 million healthcare visits annually, mainly due to bacterial vaginosis, candidiasis, and trichomoniasis (1,2). Bacterial vaginosis (BV) is the most common vaginal infection among reproductive-aged women, and it is characterized by the reduction or replacement of lactobacilli and an increase of opportunistic anaerobic bacteria (3–5). Members of the genus Lactobacillus, such as L. crispatus, L. jensenii and L. iners, are distinctive markers of a healthy vaginal microbiome, while Gardnerella vaginalis, Prevotella, Atopobium, Mobiluncus and Peptostreptococcus are frequent BV-associated anaerobic bacteria (3,6).
Oral metronidazole or vaginal clindamycin are routinely used as first-line treatment options for BV, however, several pathogenic bacteria, such as Mobiluncus and Atopobium species, are commonly resistant to those antimicrobials (3,7–10). The intermittent and long-term use of metronidazole or clindamycin, and infections with resistant bacteria, often result in high recurrence rates of BV within the first 12 months of treatment (10–12). Additionally, the increase of antimicrobial resistance in anaerobic bacteria after treatment has been also reported (2,7,8,13).
Despite the increased antimicrobial resistance and high relapse rates, the development of new drugs has been scarce, so reliance on available treatments remains necessary (11,14). Plant-derived compounds represent a potential source of therapeutic options for a variety of bacterial infections due to their reduced cost and toxicity, as well as their reduced risk of side effects (15,16). Bixa orellana L. is known in traditional medicine for its pharmacological properties, antimicrobial activity, and reduced toxicity (17–19). Thus, B. orellana constitutes a source of molecules with promising therapeutic potential that could substitute synthetic drugs used to treat BV given its antimicrobial effectiveness (19). The aim of the study was to evaluate the in vitro antimicrobial activity of B. orellana against BV-associated anaerobic bacteria and Lactobacillus spp. strains.
|
KEY MESSAGES |
|
Motivation for the study: bacterial vaginosis is a bacterial infection that frequently affects women of reproductive age. The treatment is based on synthetic antimicrobials. Bixa orellana L. possesses antimicrobial properties and could represent a potential non-synthetic therapeutic alternative. Main findings: in vitro results suggest that, methanolic extract of Bixa orellana L. leaves possesses potential antimicrobial properties against bacteria associated to bacterial vaginosis. Implications: to identify new sources with therapeutic potential, and to promote research, discovery, and characterization of non-synthetic antimicrobials. |
MATERIALS AND METHODS
Study design and Plant material
Experimental study that preliminarily assessed the in vitro antimicrobial activity of the methanolic extract of B. orellana. The leaves of B. orellana were collected in the Chanchamayo province (11.1215° S, 75.3587° W) of the Junín region of Peru, between December 2018 and January 2019. During the collection period, the daily average temperature in Junín was 10,0°C (range: 4.0°C – 15.0°C), and the daily average rainfall was 23.7 mm, with up to 100.0% of relative humidity. Collection was carried out by trained field personnel between 10:00 and 14:00. The identification of B. orellana was based on its botanical characteristics and on a simplified phytochemical analysis performed by botanical experts from the Museo de Historia Natural of the Universidad Nacional Mayor de San Marcos and from the Universidad Peruana Cayetano Heredia (UPCH), respectively. A voucher specimen was deposited at UPCH (CDCJ2018).
Extract preparation
The leaves of the B. orellana were cleaned, oven-dried during four days at 40°C, and then ground. A liter of methanol (Germany, Darmstadt, Merck; catalog number: 106009) was added to 200 g of the product, then the mixture was macerated for seven days at room temperature in an amber glass container. The container was shaken by hand once for five minutes every day. Then, the mixture was filtered using Whatman N°2 papers, and then oven-dried at 40°C until the methanol evaporated resulting in a pasty-like extract. Finally, the extract was diluted in 40% dimethyl sulfoxide (DMSO) (Germany, Darmstadt, Merck; catalog number: D9170) to yield a final concentration of 0.5 mg/µL. The extract used in the experiments was liquid and had a dark green-brown color.
Collection, cultivation, and identification of microorganisms
The antimicrobial activity of the extract was assessed against two bacterial groups; American Type Culture Collection (ATCC) reference strains, and clinical isolates.
The first group consisted of eight ATCC strains. Prevotella bivia (ATCC 29303), Peptococcus niger (ATCC 27731), Peptostreptococcus anaerobius (ATCC 27337), Mobiluncus curtisii (ATCC 43063), Atopobium vaginae (ATCC BAA-55) and Veillonella parvula (ATCC 10790) were anaerobically recovered using GasPak jars (USA, Maryland, Becton Dickinson; catalog number: 260626), while Lactobacillus crispatus (ATCC 33197) and Gardnerella vaginalis (ATCC 14018) were recovered in a microaerophilic environment (USA, Massachusetts, Thermo Fisher; catalog number: AN0025A). Bacterial cultures were performed on Columbia blood agar (Germany, Darmstadt, Merck; catalog number: 27688), Human blood tween (HBT) agar, and Man-Rogosa-Sharpe (MRS) agar (Germany, Darmstadt, Merck; catalog number: 1106600500) at 37°Cas described in previous studies (20-22). L. crispatus (ATCC 33197) was included as a reference for the Lactobacillus complex that is found in the healthy human vagina, since this study preliminarily assessed the antimicrobial activity of the extract, while the other ATCC strains were used as references of BV-associated anaerobic pathogens (3,6).
The second group consisted of twenty-two clinical isolates; eleven G. vaginalis and eleven Lactobacillus spp. These bacteria were isolated between September and December 2019 from samples obtained from written-informed adult women under an Institutional Review Board-approved research project (code: 104179) at the UPCH and Hospital Municipal de Surco in Lima, Peru. Vaginal discharge samples were collected and transported at 4°C from the Hospital to UPCH, and processed within two hours of collection. The diagnosis of BV was performed using the Amsel criteria (23), and positive samples with Lactobacillus were cultivated on MRS agar for the selective growth of lactobacilli (21). BV-positive samples were also cultured on HBT agar for the isolation of G. vaginalis (22). Plates were then incubated in GasPak anaerobic jars using anaerobic or microaerophilic generators (USA, Massachusetts, Thermo Fisher; catalog number: AN0025A) for 72 hours. G. vaginalis and Lactobacillus spp. were identified using the Neisseria Haemophilus (USA, North Carolina, Biomérieux; catalog number: 21346), and Anaerobes and Corynebacteria (USA, North Carolina, Biomérieux; catalog number: 21347) identification cards in the Vitek2 system, respectively.
Antimicrobial susceptibility testing
Disk diffusion agar method
Bacteria were suspended in 0.85% saline (8.5 g/L NaCl) and turbidity was adjusted to the McFarland standard No. 0.5. Using a sterile swab, the inoculum of Lactobacillus was spread on MRS agar, and other bacterial species was spread on Columbia blood agar. Subsequently, four wells were made on the agar with the back of a sterile 1 mL pipette tip. In addition, four sterile 6-mm paper disks (UK, Hampshire, Oxoid; catalog number: CT0998B) were placed on the agar (Figure 1). Two wells and two paper disks were used to evaluate 40% DMSO as a bacterial growth inhibitor, and the other wells and disks were used to test the antimicrobial activity of the extract.
The activity of 40% DMSO (Figure 1, on the left side of the plates) was assessed at different volumes using 1:1 dilution with saline solution; disks were soaked with 10 and 20 µL, and wells were filled with 50 and 100 µL. Correspondingly, the antimicrobial activity of the extract was assessed using the same volumes and procedures (Figure 1, on the right side of the plates). The plates were incubated at 37°C, and after three days the inhibition zones were recorded and interpreted according to the Duraffourd scale (24).
Minimum Inhibitory Concentration (MIC)
The extract at concentrations of 1, 2, 4, 8, 16, and 32 mg/mL was added to the Columbia agar supplemented with 5% sheep blood, as previously described (25). Ten microliters of a bacterial suspension with turbidity equivalent to the McFarland standard N°. 0.5 were inoculated onto each plate. The plates were incubated in an anaerobic or microaerophilic environment at 37°C, depending on the bacterial requirements for growth, and MIC was defined as the lowest concentration of the extract at which no bacterial growth was observed (25). Three technical replicates were performed for each assessment. Bacterial strains were inoculated on Columbia agar supplemented with 5% sheep blood as positive controls.
Minimum Bactericidal Concentration (MBC)
A previously described method, with some modifications, was used as reference because MBC could not be determined with a conventional liquid method since the extract had a dark color (26). The inoculated region with no bacterial growth was scraped from plates used for MIC determination with a sterile loop and then gently spread onto a new plate with non-selective agar. Based on bacterial requirements for growth, plates were incubated in either anaerobic or microaerophilic conditions at 37°C for 72 hours. The MBC was defined as the lowest extract concentration at which no bacterial growth was observed. Three technical replicates were performed for each MBC determination.
Statistical Analysis
Technical triplicates had no significant variation; therefore, data were reported using averages ± standard deviations (SD). The MIC50, MIC90, MBC50 and MBC90 were estimated for the group of clinical isolates. Lastly, the MIC and MBC differences between clinical isolates of G. vaginalis and Lactobacillus were evaluated using the Mann-Whitney U test. Data analysis was performed in Stata v15 (StataCorp., College Station, TX, USA) considering a value of p<0.050 as significant.
RESULTS
The 40% DMSO, regardless of the volume used, had no bactericidal effect neither inhibited the bacterial growth of the ATCC reference strains used. Figure 1 (left side of the plates) shows the results for some ATCC reference strains.
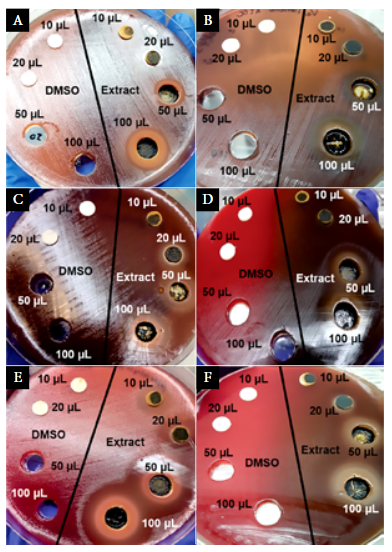
Figure 1. Antimicrobial susceptibility testing using 40% DMSO and B. orellana extract against anaerobic bacteria; a) Prevotella bivia ATCC 29303, b) Veillonella parvula ATCC 10790, c) Atopobium vaginae ATCC BAA-55, d) Mobiluncus curtisii ATCC 43063, e) Peptoestreptococcus anaerobius ATCC 27337, and f) Peptococcus niger ATCC 27731. Sterile paper disks and wells on the left side of the plates were used for testing the activity of 40% DMSO, and those on the right side were used for testing the extract.
Antimicrobial activity against ATCC reference strains
The inhibition zone was directly related to the volume of extract used (Table 1). The extract exhibited high antimicrobial activity against all ATCC reference strains, except for L. crispatus ATCC 33197, P. bivia ATCC 29303, and V. parvula ATCC 10790 (Table 1). Interestingly, the growth of L. crispatus ATCC 33197 was not inhibited regardless of the volume used, and the growth of V. parvula ATCC 10790 was only inhibited by 100 µL of the extract.
Table 1. In vitroactivity of B. orellana L. extract against ATCC reference strains.
|
ATCC Bacteria |
Inhibition zone (mm) |
MIC |
MBC |
|||
|
10 µl a |
20µl a |
50 µl a |
100µl a |
|||
|
G. vaginalis (ATCC 14018) |
14.0 ± 1.0 |
20.3 ± 1.5 |
24.7 ± 0.6 |
30.3 ± 0.6 |
1.0 |
4.0 |
|
L. crispatus (ATCC 33197) |
0.0 ± 0.0 |
0.0 ± 0.0 |
0.0 ± 0.0 |
0.0 ± 0.0 |
32.0 |
32.0 |
|
A. vaginae (ATCC BAA-55) |
0.0 ± 0.0 |
12.0 ± 1.0 |
14.0 ± 1.0 |
18.3 ± 0.6 |
4.0 |
4.0 |
|
M. curtisii (ATCC 43063) |
0.0 ± 0.0 |
12.3 ± 1.2 |
18.3 ± 0.6 |
20.0 ± 1.0 |
4.0 |
8.0 |
|
P. niger (ATCC 27731) |
13.3 ± 0.6 |
15.0 ± 1.0 |
22.3 ± 0.6 |
25.0 ± 0.0 |
8.0 |
8.0 |
|
P. anaerobius (ATCC 27337) |
13.7 ± 1.5 |
18.7 ± 0.6 |
25.7 ± 1.2 |
28.3 ± 0.6 |
4.0 |
8.0 |
|
P. bivia (ATCC 29303) |
0.0 ± 0.0 |
10.3 ± 0.6 |
12.0 ± 1.0 |
18.7 ± 1.2 |
32.0 |
32.0 |
|
V. parvula (ATCC 10790) |
0.0 ± 0.0 |
0.0 ± 0.0 |
0.0 ± 0.0 |
12.3 ± 1.5 |
32.0 |
>32.0 |
a Mean ± standard deviation
The MIC and MBC for ATCC stains are reported in Table 1. G. vaginalis ATCC 14018 was the most susceptible bacteria, with MIC and MBC of 1.0 mg/mL and 4.0 mg/mL, respectively. P. bivia ATCC 29303, V. parvula ATCC 10790 and L. crispatus ATCC 33197 were the most resistant bacteria, displaying higher MIC and MBC values compared to other ATCC strains.
Antimicrobial activity against clinical isolates
Among G. vaginalis clinical isolates, the most susceptible was strain M10 (MIC and MBC: 1.0 mg/mL), followed by strains MD (MIC: 1.0 mg/mL; MBC: 4.0 mg/mL) and M23 (MIC and MBC: 2.0 mg/mL) while the other eight isolates displayed MIC and MBC values of 2.0 mg/mL and 4.0 mg/mL, respectively (Table 2). Overall, the MIC50 and MIC90 were 2 mg/mL, and the MBC50 and MBC90 were 4.0 mg/mL.
Lactobacillus spp. isolates grew abundantly over the range of 1.0 to 16.0 mg/mL of the extract. However, there was no evidence of bacterial growth at 32.0 mg/mL. Consequently, the MIC value for all clinical isolates of Lactobacillus spp. was 32.0 mg/mL (Table 2), and the MIC50 and MIC90 were 32.0 mg/mL. Regarding the MBC, 54.5% (6/11) of the Lactobacillus spp. isolates (M7, M24, M25, M33, MA and MB; Table 2) showed a value of 32.0 mg/mL, while other clinical isolates showed values above 32.0 mg/mL. The MBC50 and the MBC90 for Lactobacillus spp. were 32.0 mg/mL and >32.0 mg/mL, respectively.
Table 2. Minimum inhibitory (MIC) and minimum bactericidal concentration (MBC) in ATCC reference strains and clinical isolates of G. vaginalis and Lactobacillus spp.
|
Bacteriaa |
MIC |
MBC |
|
|
G. vaginalis |
|
|
|
|
|
M10 (n=1) |
1.0 mg/mL |
1.0 mg/mL |
|
|
ATCC 14018 and MD (n=2) |
1.0 mg/mL |
4.0 mg/mL |
|
|
M23 (n=1) |
2.0 mg/mL |
2.0 mg/mL |
|
|
MG, M5, M8, M9, M26, M29, M30 and M31 (n=8) |
2.0 mg/mL |
4.0 mg/mL |
|
Lactobacillus spp. |
|
|
|
|
|
ATCC 33197, M7, M24, M25, M33, MA and MB (n=7) |
32.0 mg/mL |
32.0 mg/mL |
|
|
M1, M3, M4, M6 and M27 (n=5) |
32.0 mg/mL |
>32.0 mg/mL |
a Clinical isolates were coded as: M#, MD, MG, MA, or MB
The extract exhibited a significant and differential antimicrobial activity between G. vaginalis and Lactobacillus spp. strains (p<0.001). Specifically, G. vaginalis isolates were inhibited at low concentrations of the extract (MIC: 2.0 mg/mL, MBC: 4.0 mg/mL), while Lactobacillus spp. isolates were inhibited at higher concentrations (MIC: 32.0 mg/mL, MBC: 3.0 mg/mL).
DISCUSSION
In recent years, the use of medicinal plants or herbs has been promoted by scientific research due to their pharmacological activity, low toxicity, and inexpensive accessibility (15,16,19,25). Plants possess and produce a wide variety of secondary metabolites such as tannins, terpenoids, flavonoids, glycosides, saponins, anthranoids, quinones and coumarins, to which antimicrobial properties have been attributed (27). Several studies suggest that B. orellana possesses various properties, including antimicrobial, antifungal, antioxidant, anti-inflammatory, and analgesic activity (19, 28). The antimicrobial activity of B. orellana against several microorganisms has been previously described (19,28), however, its antimicrobial activity on microaerophilic or anaerobic bacteria responsible for BV has been scarcely described.
Our results suggest that, in the absence of bacteriostatic or bactericidal activity of the 40% DMSO, the observed antimicrobial activity can be attributed to the extract. This finding is consistent with several other studies that suggest that B. orellana leaves extracts, obtained by methanol- or ethanol-based techniques, have antimicrobial activity against reference ATCC strains and clinical isolates (19,29,30). Therefore, based on our results and the results reported by other studies (19,28-30), it is reasonable to consider that the evaluated extract possesses antimicrobial activity.
BV is a dysbiosis characterized by drastic changes in the biota of the vaginal tract that result in a replacement of the lactobacillus-predominant vaginal flora by anaerobic bacteria (3,6). G. vaginalis is one of the most frequent anaerobic bacteria causing BV, which symbiotically can form a polymicrobial biofilm with several BV-associated anaerobic bacteria such as A. vaginae and Prevotella spp (2,6,31). Other anaerobic bacteria commonly detected in women with BV are Peptoestreptococcus and Mobiluncus (6,32,33). Based on the observed low MIC and MBC values, it can be inferred that the evaluated extract has a high antimicrobial activity against most of the ATCC reference strains used here and also against clinical isolates of G. vaginalis. However, it is important to note that P. bivia and V. parvula, both ATCC strains, displayed low susceptibility to the extract. To the best of our knowledge, the in vitro activity of the B. orellana extract against G. vaginalis and other anaerobic pathogens has been scarcely described. Further studies are needed to validate our findings in a wide variety of clinical isolates.
Vaginal lactobacilli regulate pH and protect the mucosa against the establishment of pathogenic microorganisms (3,33). In this study, L. crispatus ATCC 33197 and clinical isolates of Lactobacillus spp. were used as references of the Lactobacillus complex found in the healthy human vagina (6,32). Interestingly, the extract inhibited the growth of lactobacilli at concentrations of 32.0 mg/mL. However, our results differ from those described elsewhere. Galindo-Cuspinera et al. (34) suggested that the extract obtained from fruits and seeds of B. orellana exhibited limited antimicrobial activity against L. lactis ATCC 11454 and L. casei ATCC 39539, and no activity against L. plantarum ATCC 700210. Similarly, Ogunshe et al. (35) suggested that B. orellana had limited in vitro activity against various vaginal Lactobacillus spp. strains. Overall, these discrepant results could be explained by; i) the use of different extraction methods (i.e., type of extract and solvent) resulting in variable concentration of phytochemicals, ii) the use of different parts of B. orellana (i.e., leaves, seeds, pods or fruits) for extract preparation, iii) the geographical location and exposure to climatic and environmental factors affecting B. orellana, and iv) by intrinsic differences between Lactobacillus spp. strains used in other studies (19).
Overall, our findings are consistent with several other studies suggesting that B. orellana extract possesses antimicrobial activity (17–19,28,30). An important major strength of this in vitro study is the evaluation of the antimicrobial activity of the extract against anaerobic bacteria representative of those causing BV, and against representative bacteria of the healthy vaginal biota. Our findings may support the scale-up and innovation of research for the development of potential therapeutic options. However, it is also important to consider the limitations of this in vitro study. Multiple phytochemicals are found in B. orellana, including flavonoids, polyphenols, tannins, quinones, terpenoids and alkaloids, which exert antimicrobial activity through complex processes (19). In this preliminary study we have not characterized the phytochemical compounds or their concentrations; therefore, we could not establish nor isolate the individual antimicrobial activity of each component of the extract against the studied bacterial isolates. Furthermore, it is reasonable to assume that the antimicrobial activity described here may not be extrapolated to all B. orellana species given possible intrinsic differences among them and differences in plant cultivation.
In summary, our in vitro experiments suggest that the extract of B. orellana could be a source of antimicrobial compound or compounds with high selective antimicrobial activity against most BV-associated anaerobic bacteria, and with reduced activity against protective bacteria found in the healthy vaginal mucosa. Further research is needed to identify the compound or compounds with antimicrobial activity, in order to validate the preliminary differential activity described in our study by using a more rigorous and complex study design.
Funding: The study was supported by INNOVATEPERU (030-INNOVATEPERU-ITAI-2016). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions: JM, SL, and JT conceptualized, designed the methodology, conducted the research, analyzed and interpreted the data, and drafted the manuscript. LR assisted with data analysis and interpretation. JT managed funding. JT and OT provided research resources. All authors approved the final version of the manuscript and assume responsibility for the article.
Conflicts of interest: The authors declare that they do not have conflicts of interest
References
1. Prabhu A, Gardella C. Common vaginal and vulvar disorders. Med Clin North Am. 2015;99(3):553-574. doi: 10.1016/j.mcna.2015.01.008.
2. Javed A, Parvaiz F, Manzoor S. Bacterial vaginosis: An insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microb Pathog. 2019;127:21-30. doi: 10.1016/j.micpath.2018.11.046.
3. Coudray MS, Madhivanan P. Bacterial vaginosis-A brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol. 2020;245:143-148. doi: 10.1016/j.ejogrb.2019.12.035.
4. van Schalkwyk J, Yudin MH. Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can. 2015;37(3):266-274. doi: 10.1016/S1701-2163(15)30316-9.
5. Abdul-Aziz M, Mahdy MAK, Abdul-Ghani R, Alhilali NA, Al-Mujahed LKA, Alabsi SA, et al. Bacterial vaginosis, vulvovaginal candidiasis and trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana’a city, Yemen. BMC Infect Dis. 2019;19(1):879. doi: 10.1186/s12879-019-4549-3.
6. Onderdonk AB, Delaney ML, Fichorova RN. The Human Microbiome during Bacterial Vaginosis. Clin Microbiol Rev. 2016;29(2):223-238. doi: 10.1128/CMR.00075-15.
7. Tomás M, Palmeira-de-Oliveira A, Simões S, Martinez-de-Oliveira J, Palmeira-de-Oliveira R. Bacterial vaginosis: Standard treatments and alternative strategies. Int J Pharm. 2020;587:119659. doi: 10.1016/j.ijpharm.2020.119659.
8. Petrina MAB, Cosentino LA, Rabe LK, Hillier SL. Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin. Anaerobe. 2017;47:115-119. doi: 10.1016/j.anaerobe.2017.05.005.
9. Lopes dos Santos Santiago G, Grob P, Verstraelen H, Waser F, Vaneechoutte M. Susceptibility testing of Atopobium vaginae for dequalinium chloride. BMC Res Notes. 2012;5:151. doi:10.1186/1756-0500-5-151.
10. Faught BM, Reyes S. Characterization and Treatment of Recurrent Bacterial Vaginosis. J Womens Health (Larchmt). 2019;28(9):1218-1226. doi: 10.1089/jwh.2018.7383.
11. Larsson PG, Forsum U. Bacterial vaginosis--a disturbed bacterial flora and treatment enigma. APMIS. 2005;113(5):305-316. doi: 10.1111/j.1600-0463.2005.apm_113501.x.
12. Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478-1486. doi: 10.1086/503780.
13. Beigi RH, Austin MN, Meyn LA, Krohn MA, Hillier SL. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am J Obstet Gynecol. 2004;191(4):1124-1129. doi: 10.1016/j.ajog.2004.05.033.
14. Bradshaw CS, Sobel JD. Current Treatment of Bacterial Vaginosis-Limitations and Need for Innovation. J Infect Dis. 2016;214Suppl 1:S14-S20. doi: 10.1093/infdis/jiw159.
15. Cheesman MJ, Ilanko A, Blonk B, Cock IE. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn Rev. 2017;11(22):57-72. doi: 10.4103/phrev.phrev_21_17.
16. Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv. 2015;33(8):1582-1614. doi: 10.1016/j.biotechadv.2015.08.001.
17. Vilar Dde A, Vilar MS, de Lima e Moura TF, Raffin FN, de Oliveira MR, de Oliveira-Franco CM, et al. Traditional uses, chemical constituents, and biological activities of Bixa orellana L.: a review. ScientificWorldJournal. 2014;2014:857292. doi: 10.1155/2014/857292.
18. Stohs SJ. Safety and efficacy of Bixa orellana (achiote, annatto) leaf extracts. Phytother Res. 2014;28(7):956-960. doi: 10.1002/ptr.5088.
19. Coelho Dos Santos D, Silva Barboza AD, Ribeiro JS, Rodrigues Junior SA, Campos ÂD, Lund RG. Bixa orellana L. (Achiote, Annatto) as an antimicrobial agent: A scoping review of its efficiency and technological prospecting. J Ethnopharmacol. 2022;287:114961. doi: 10.1016/j.jep.2021.114961.
20. Rosca AS, Castro J, Cerca N. Evaluation of different culture media to support in vitro growth and biofilm formation of bacterial vaginosis-associated anaerobes. PeerJ. 2020;8:e9917. doi: 10.7717/peerj.9917.
21. Pendharkar S, Magopane T, Larsson PG, de Bruyn G, Gray GE, Hammarström L, Marcotte H. Identification and characterization of vaginal lactobacilli from South African women. BMC Infect Dis. 2013;13:43. doi: 10.1186/1471-2334-13-43.
22. Totten PA, Amsel R, Hale J, Piot P, Holmes KK. Selective differential human blood bilayer media for isolation of Gardnerella (Haemofilus) vaginalis. J Clin Microbiol. 1982;15(1):141-147. doi: 10.1128/JCM.15.1.141-147.1982.
23. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14-22. doi: 10.1016/0002-9343(83)91112-9.
24. Duraffourd C, D’ Hervicourt L, La Praz JC. Cuaderno de Fitoterapia Clínica. Francia: Editorial Masson; 1983.
25. Klancnik A, Piskernik S, Jersek B, Mozina SS. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods. 2010;81(2):121-126. doi: 10.1016/j.mimet.2010.02.004.
26. Mogana R, Adhikari A, Tzar MN, Ramliza R, Wiart C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med Ther. 2020;20(1):55. doi: 10.1186/s12906-020-2837-5.
27. Patra AK. An Overview of Antimicrobial Properties of Different Classes of Phytochemicals. Dietary Phytochemicals and Microbes. 2012;1-32. doi: 10.1007/978-94-007-3926-0_1.
28. Poma-Castillo L, Espinoza-Poma M, Mauricio F, Mauricio-Vilchez C, Alvítez-Temoche D, Mayta-Tovalino F. Antifungal Activity of Ethanol-extracted Bixa orellana (L) (Achiote) on Candida albicans, at Six Different Concentrations. J Contemp Dent Pract [Internet]. 2019 [citado el 9 de diciembre de 2022]; 20(10): 1159-1163. Disponible en: https://thejcdp.com/doi/JCDP/pdf/10.5005/jp-journals-10024-2672.
29. Kar B, Chandar B, Rachana SS, Bhattacharya H, Bhattacharya D. Antibacterial and genotoxic activity of Bixa orellana, a folk medicine and food supplement against multidrug resistant clinical isolates. J Herb Med. 2022;32:100502. doi: 10.1016/j.hermed.2021.100502.
30. Banadkoki AZ, Kouhsari E, Amirmozafari N, Roudbary M, Nasrabadi MRB. Antibacterial, antifungal and cytotoxic activities of some medicinal plants against multidrug resistance pathogens. Rev Med Microbiol. 2018;29:182–188. doi: 10.1097/MRM.0000000000000146.
31. Rosca AS, Castro J, Sousa LGV, Cerca N. Gardnerella and vaginal health: the truth is out there. FEMS Microbiol Rev. 2020;44(1):73-105. doi: 10.1093/femsre/fuz027.
32. Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818.
33. Diop K, Dufour J-C, Levasseur A, Fenollar F. Exhaustive repertoire of human vaginal microbiota. Hum Microbiome J. 2019;11:100051. doi: 10.1016/j.humic.2018.11.002.
34. Galindo-Cuspinera V, Westhoff DC, Rankin SA. Antimicrobial properties of commercial annatto extracts against selected pathogenic, lactic acid, and spoilage microorganisms. J Food Prot. 2003;66(6):1074-1078. doi: 10.4315/0362-028x-66.6.1074.
35. Ogunshe AA, Lawal OA, Iheakanwa CI. Effects of simulated preparations of plants used in Nigerian traditional medicine on Candida spp. associated with vaginal candidiasis. Ethnobot Res Appl [Internet]. 2008 [citado el 2 de octubre de 2021]; 6:373–383. Disponible en: https://ethnobotanyjournal.org/era/index.php/era/article/view/165.
Cite as: Marcas J, Romero L, Tipiani O, Loyola S, Tamariz J. In vitro antimicrobial activity of Bixa orellana L. leaves extract against bacterial vaginosis-associated anaerobic bacteria and Lactobacillus spp. Rev Peru Med Exp Salud Publica. 2022;39(4):408-14. doi: https://doi.org/10.17843/rpmesp.2022.394.11978.
Correspondence: Steev Loyola; steev.loyola@gmail.com
Received: 11/08/2022
Approved: 14/12/2022
Online: 27/12/2022