Original
Article
Lymphocyte subsets, dendritic cells and cytokine profiles in mice with melanoma treated with Uncaria tomentosa
Iván Lozada-Requena1,2,a, César Núñez 1,2,b, Yubell Alvárez1,c, Laura Kahn1,d, José Aguilar1,e
1 Immunology laboratory. Departament of cellular and molecular sciences. Faculty of sciences an philosophy. Universidad Peruana Cayetano Heredia. Lima, Peru.
2 EMINDES SAC (Empresa de Investigación y
Desarrollo en Cáncer). Lima, Perú.
a Master in Science, b physican; c
biologist; d degree of bachelor in biology; e
rheumatologist specializing in immunology
ABSTRACT
Objectives. To evaluate the immunomodulatory effect on lymphocyte subsets, dendritic cells (DC), Th1 / Th2 / Th17 and inflammatory cytokines on systemic level and/or in the tumor microenvironment of mice with or without melanoma. Materials and methods: Peripheral blood and/or primary tumors samples were obtained of mice with B16 melanoma treated or not with a hydroalcoholic extract of Uncaria tomentosa (UT) with 5.03% of pentacyclic oxindole alkaloids (UT-POA) obtained from the bark of the plant. All cell assays and cytokine measurements were performed by flow cytometry. Results. UT-POA systemically increased CD4/CD8a relation while cell activation was inversely proportional; increased the proportion of DCm; induced a pro-inflammatory Th1 profile and reduced Th17 response. TNF-α and IL-17A positively and negatively correlated with CD4/CD8a relation. Conclusions. The increase of Th1 (TNF-α) may result in the increase of CD4 or M1 macrophage activation. Although UT-POA shows increased DCm, is not dose-dependent. Th17(IL-17A) decreased can support the function of CD8a lymphocytes. UT-POA shows better systemic immunomodulatory effects than intratumoral.
Key words: Uncaria tomentosa; Lymphocyte activation; Dendritic cells; Melanoma (source: MeSH NLM).
INTRODUCTION
Malignant melanoma, a tumor derived from skin melanocytes, is the deadliest form of skin cancer (1). The increased incidence of human melanoma is related to hereditary or ethnic factors, environmental factors such as UV exposure, and nutritional and lifestyle factors (2). Murine models such as melanoma B16 cells in BALB/c and C57BL/6 mice are accepted among scientists because of the understanding of the genetics and because they reproduce characteristics of human cancer (3). Therefore, murine models are used to evaluate drugs, immunotherapies, and medicinal plants.
Medicinal plants have few side effects, low
cytotoxicity, and the ability to act on target cells without affecting normal
cells, and they are also a low-cost therapeutic option. Research on medicinal
plants has yielded encouraging results. Park W.-B. et al. evaluated the effect of Viscum album (mistletoe) lectins on mice with melanoma, and observed proapoptotic, antiangiogenic, antimetastatic, and survival-enhancing
effects (4). Currier N.L. et al. showed upregulation of
natural killer (NK) cells in mice with leukemia that were treated with Echinacea
purpurea (Echinacea) (5). Epigallate polyphenol (EG) derived from Camelia sinensis
(green tea) has an antimetastatic activity. Likewise, epigallocatechin-3-gallate
(EGCG) and dacarbazine reduce metastases and tumor growth in mice with
melanoma (6).
Uncaria tomentosa (UT; Cat’s claw) is a vine from the Peruvian Amazon jungle that has anti- inflammatory, antioxidant, immunomodulatory, and antiproliferative properties (7,8). Using the same extract as
in our present study, Dreifuss et al. showed antitumor and antioxidant
effects in Walker-256 rats with carcinosarcoma (9). The
antiproliferative and antitumor activity of UT has also been shown in cell
lines of breast cancer (MT-3) and Ewing’s sarcoma (MHH-ES-a) (10).
Although there are not many studies on humans, one in
particular stands out, which shows an
improvement in the quality of life of patients with solid tumors (11)
and another one that shows alleviation of neutropenia and repair of the DNA
damage related to chemotherapy (12). UT contains quinovic acid
glycosides, steroids, polyphenols, polyhydric triterpenes, proanthocyanidins,
catechins, saponins, tannins, sterols, flavonoids, and alkaloids. The most clinically relevant effects occur when these compounds act synergistically (13). Sheng et al. showed that C-MED100 (a UT extract with less than 0.05%
of oxindole alkaloids) increases the numbers of T lymphocytes (TL) in rats with
doxorubicin-induced leukopenia (14). It also promotes repair of
splenocyte DNA in UV- irradiated rats (15). Aguilar et al.
and Aquino et al. showed that ethanolic and methanolic UT extracts reduce inflammation in
plantar edema (7,16). Sandoval et al. showed that inhibition of inflammation is associated with
downregulation of TNF-α and is mediated by inhibition of NF-kB activity (17).
Fazio et al. showed that UT reduces melanoma tumors, lung metastases, and
downregulates TNF-α and IL-6 (8).
Regarding inflammation and cancer, it is still
unknown whether it is the state of chronic inflammation that leads to the
development of neoplastic cells or vice versa (18). Coussens et
al. demonstrated the presence of growth factors, TNF- α, and reactive
oxygen species (ROS) in the tumor microenvironment (TME); ROS damage DNA and
initiate neoplasms (19). Tumor immunotherapy induces cell-mediated
immunity (for example, dendritic cell [DC] vaccines or activation of CD4+ TLs
involved in the direct protection against the development of tumors and in
tumor regression) (20). Likewise, upregulation of IL-2 because of
tumors causes a dose-dependent decrease in tumor mass (21), and it
has been observed that PBI-1393 is capable of reducing the size of tumors and
can stimulate lymphocytes to produce antitumor interferon (IFN) and IL-2 (22).
Everything indicates that TLs play a crucial role in the defense of the host against the disease. CD4+ TLs differentiate into the following subsets: Th1, Th2, Th17, Th22, Treg,
Th9, and Th follicular helper cells (23). Nonetheless, the Th1/Th2
system has been an immunological paradox for a long time. Th1 (IFN-y and IL-2) or proinflammatory cytokines promote a response to intracellular pathogens. Th1 lymphocytes recognize tumor antigens, whether directly
(some melanomas express on-site MHC II) or via DC cross-presentation mechanisms (24). It is possible
that Th1 immune cells, through IFN- y generate an antitumor response in
which CD8+ TLs do not participate directly because they activate NKs, macrophages, and monocytes involved
in the defense against neoplasms (25),
while Th2 anti-inflammatory cytokines (IL4, IL-5, and IL-10) stimulate
elimination of extracellular parasites and induce polarization of
tumor-associated macrophages (TAMs) or M2d that tend to promote progression of
cancer (26). On the other hand, TAMs or M2d are also reported to
play an anticancer role because they can indirectly activate the phagocytic
system to release ROS and nitric oxide synthase (NOS) that are cytotoxic to tumor cells (27).
There are in vivo and in vitro melanoma
studies that show Th2 directionality (28). The other newly
discovered subset is Th17, which is generated in the presence of TGF-β and IL-6 and proliferates under
the influence of IL-23 (29). During the development of tumors, Th17
cells were found in prostate cancer and in the TME (30). The
produced cytokines, including IL17, have controversial functions. On the one
hand, they impair the function of CD8+ TLs and promote carcinogenesis and
neovascularization of tumors via STAT3; on the other hand, they have been
reported to perform antitumor functions. Because they may cause inflammation and destruction of tissue, they are potentially useful for cancer treatment.
IL-17 induces inflammatory cytokines and chemokines close to the TME, which can
facilitate the recruitment of DCs specialized in presentation of tumor antigens
as well as cytotoxic effector cells such as CD8+ TLs, NKs, and NKT cells that
can attack the tumor (31). DCs have several subsets, the most
important of which are myeloid DCs (mDCs) and plasmacytoid dendritic cells (pDCs). They are both
important to an antitumor response (33).
All these data suggest that immunocompetent cell populations, the Th1/Th2/Th17 balance, and inflammatory cytokines have a strong influence on the development of cancer. All the evidence indicates a correlation, but the specific role of cytokines and the complexity of their interactions have not been identified yet. Therefore, in this study, we evaluated the modulatory effect of a UT extract on lymphocyte populations and DCs and the profile of inflammatory and Th1/Th2/Th17 cytokines systemically and in the TME in a murine melanoma model.
MATERIALS AND METHODS
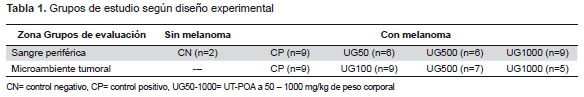
EXPERIMENTAL DESIGN
ixty-two mice were used, subdivided into 4 or 5 experimental groups according to the type of evaluation (Table 1). They received a UT hydroalcoholic extract with 5.03% pentacyclic oxindole alkaloids (UT-POA) or distilled water (negative control, CN) via a gastric tube 7 days prior to the inoculation of B16 cells, and during 22 days in the case of peripheral blood (PB) evaluation or until the tumor reached 4.5 mm for the TME evaluation.
Uncaria tomentosa (UT)
We used a hydroalcoholic extract, which was obtained from the bark of UT, in the form of a fine reddish brown powder. This extract was kindly provided by Peruvian Heritage® and prepared by decoction with ethanol and water in the
70:30 ratio for 1 hour at 20°C; subsequently, it was spray dried. The extract
contained 5.03% of pentacyclic oxindole alkaloids, quantified by high-performance
liquid chromatography (HPLC),
as described
ANIMALS
C57BL/6 mice were obtained, randomized as females
and males, from the Cayetano Heredia Peruvian University Vivarium. The experiments were performed on
groups with 6 mice on average (7 to 9 weeks old and weighing 20–25 g). They were given standard balanced food and water ad libitum.
The UT-POA extracts and distilled water were administered daily via a gastric
tube (Table 1).
B16 CELL CULTURE
The B16/BL6 (murine melanoma) cell line was cultured in a humidified atmosphere containing 5% of CO2 at 37°C in the RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 µg/mL), and 0.01% sodium pyruvate. After the cells reached >70% confluence, they were trypsinized, washed, counted, and resuspended in PBS pH 7.2 for further use.
INDUCTION OF A TUMOR
On day 22, PB samples were obtained via cardiac
puncture (approximately 1 mL) and the blood of 2 to 3 animals was pooled by diluting the blood in equal parts with complete RPMI 1640 (Sigma, St. Louis, MO, USA). In the case of a solid tumor (4.5 mm), it was
dissected, fragmented mechanically, digested with 1% type I collagenase (Gibco, USA) for 30 minutes at
37°C, and diluted with equal volume of complete RPMI 1640. Density gradient
centrifugation was performed on both types of samples with tubes containing 3
or 1 mL of 1.083 g/mL Histopaque (Sigma), respectively. They were centrifuged
at 1800 rpm at room temperature (RT) for 30 minutes, without braking. The cell ring was recovered, washed twice with
15 mL of complete RPMI 1640 at RT during the first wash and at 4°C during the
second wash at 2500 rpm for 10 minutes. The cells were counted in a Neubauer
chamber, 1:1 ratio with Trypan blue (Sigma). Once the PBMCs or TMMCs were isolated, 5 x 104 cells were plated for each 100 µL of a suspension in
sterile tubes, and they were incubated at 37°C and 5% CO2 in complete RPMI 1640 for 24 hours. The culture
supernatants (CSs) were collected to quantify the cytokines, and the cells were
washed and resuspended in a cell wash solution (1% fetal bovine serum [Hyclone]
in PBS [pH 7.4]).
Specific fluorochrome-labeled monoclonal antibodies were used: anti-CD3-PerCP, anti-CD4-FITC, anti-CD8a-PE, anti-CD44-APC, anti-CD69-PerCP, anti-CD8a-APC, anti-CD11b-FITC, anti-I-Ad-PE, and anti-CD11c-APC, and their respective isotype controls (Becton Dickinson, San Jose, CA, USA). The titration, compensation, and acquisition of antibody data were performed on a FACSCantoTM II flow cytometer (BD Immunocytometry Systems, USA). The results were analyzed in the Flow Jo software v.X10.0.7r2.
In all cases, the respective combinations of four antibodies, according to the population or populations to be identified, were added to tubes that contained the cells. They were incubated for 30 minutes at 2–8°C and washed twice with 1 mL of the cell wash solution with centrifugation at 1800 rpm for 5 minutes. Finally, the cell pellets were resuspended in 500 μL of the cell fixative (1% paraformaldehyde in pH 7.4 PBS) and analyzed on the flow cytometer.
FLOW CYTOMETRY FOR ANALYSIS OF CYTOKINES VIA CBA (CYTOMETRIC BEAD ARRAY)
Cytokine assay kits for cytometry (BD CBA Mouse Th1/Th2/Th17 and Inflammation kits, USA) were used for quantitative analysis of Th1 cytokines (IFN-y IL-2), Th2 (IL-4, IL-6, IL-10), Th17 (IL-17A), and proinflammatory cytokines (IL-6, IL-10, MCP-1 [monocyte chemoattractant protein], IFN-γ, TNF-α, and IL-12p70). The CSs were processed in strict accordance with the manufacturer’s instructions. Data were acquired on a FACSCanto™ II flow cytometer (BD Immunocytometry Systems, USA) and analyzed in the BD™ Cytometric Bead Array Software, version 1.4.
STATISTICAL ANALYSIS
The results are presented as mean ± SEM (standard error of the mean). We performed the one-tailed t test and one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc mean comparison test and Pearson’s one- tailed correlation test in the GraphPad Prism software, version 6.00 for Mac (GraphPad Software, La Jolla California USA). Differences with p < 0.05 were considered significant.
ETHICAL CONSIDERATIONS
All experiments on animals were conducted in accordance with the Principles of Laboratory Animal Care (Publication NIH 85-23, revised in 1985). The project was approved by the Institutional Ethics Committee of Cayetano Heredia Peruvian University, registration code 56290 (SIDISI).
RESULT
CD4+/CD8a+ RATIO AND LYMPHOCYTE ACTIVATION IN PB AND TME
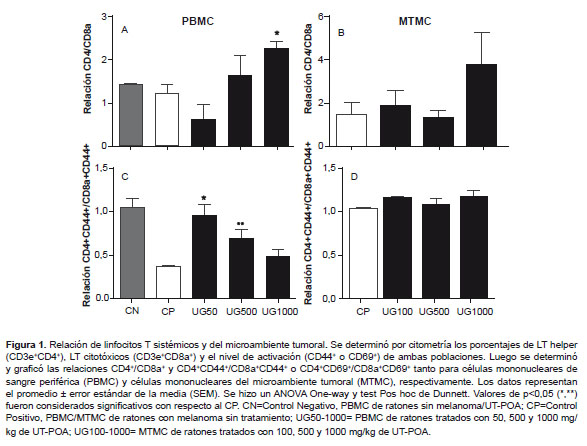
The percentages of helper T cell (CD3+CD4+) populations and cytotoxic cells (CD3+CD8a+) were determined, and the CD4/CD8a ratio was calculated. Additionally, the activation level was measured for both TL populations with two markers that may be used interchangeably, CD44 and CD69. The subpopulations in groups that were treated with UT-POA or untreated were analyzed for PBMCs (UT-POA dose: 50, 500, or 1000 mg/kg) and for the TMMCs (UT- POA dose: 100, 500, or 1000 mg/kg). The results indicated that the systemic CD4/CD8a ratio increased significantly in response to 1000 mg/kg UT-POA (Figure 1A), while the systemic cell activation (judging by the CD4+CD44+/CD8a+CD44+ ratio) was significant in response to the doses 50 and 500 mg/kg. Although at 1000 mg/kg, we saw stronger activation than in the positive control (PC), the effect was not significant (Figure 1C). In the TME, no significant changes were observed either in the CD4/CD8a or in the activation ratio of CD4+CD69+/CD8a+CD69+ (Figure 1B and 1D).
IMMUNOCOMPETENT POPULATIONS IN THE TME
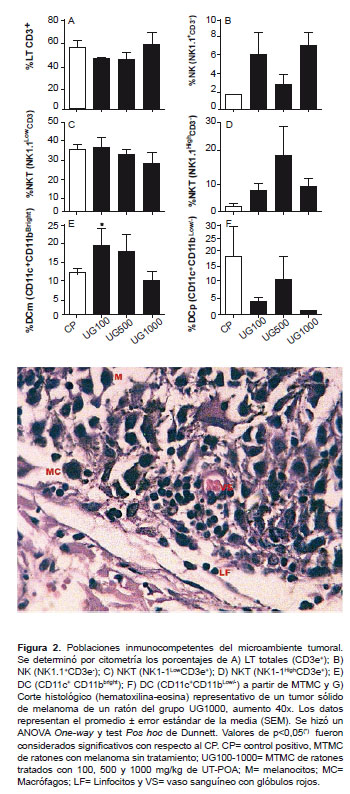
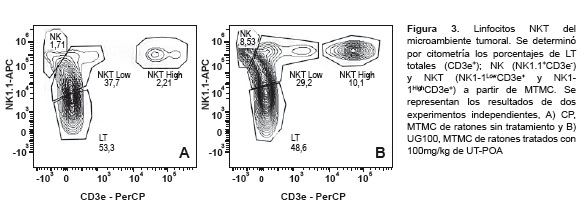
We determined the percentages of total TLs (CD3+); NK lymphocytes (NK1.1+CD3-); NKT lymphocytes (NK1.1+CD3+) and myeloid dendritic cells (mDC, CD11c+CD11bbrigth); and plasmacytoids (pDC, CD11c+CD11blow/-) in the TME of mice either treated or not treated with UT-POA (100, 500, or 1000 mg/kg). The results revealed that none of the TL, NK, or NKT populations showed significant variations in response to the UT-POA treatment (Figures 2A-D , Figure 3). Likewise, we found that UT-POA at 100 mg/kg significantly increased the percentage of mDCs, while the pDCs showed no changes (Figura 2E and F). The presence of immunocompetent cells was detected histologically in the TME (Figure 2G).
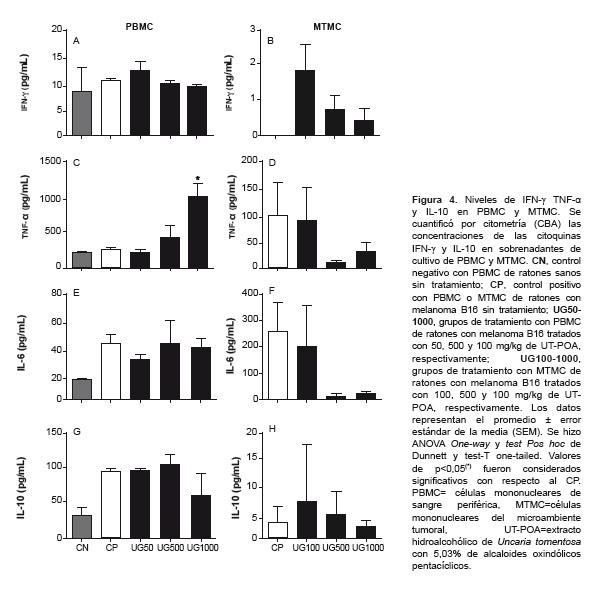
SYSTEMIC AND TME CYTOKINES
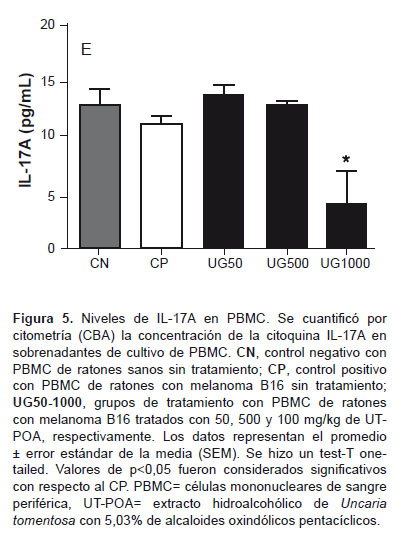
The concentration of Th1 (IFN-y, TNF-α) and Th2 (IL-6, IL-10) cytokines were determined via cytometry (CBA) in the PBMC and TMMC supernatant; Th17 (IL-17A) in PBMCs; Th1 (IL-2) and Th2 (IL-4) in PBMCs; IL-12p70 and MCP-1 in TMMCs. Upregulation of TNF-α was detected (Figure 4C) as well as significant downregulation of IL-17A in PBMCs at 1000 mg/kg UT-POA (Figure 5). There were no significant differences in IL-2 and IL-4 (PBMCs) or in IL-12p70 and MCP-1 (TMMC; data not shown).
CORRELATION BETWEEN TLs AND SYSTEMIC CYTOKINES
The correlations among the CD4/CD8a ratio of TLs, TNF-α, and IL-17A were evaluated. The results indicated that there was a positive correlation (r = 0.8559) between CD4/CD8a and TNF-α and a negative correlation (r = 0.8344) between CD4/CD8a and IL-17A. In both cases, the correlations were significant (p < 0.05) (Figure 6).
DISCUSSION
This is the first study to evaluate the effects of a UT extract on the TME. There have been previous studies on the TME only to assess the oxidative parameters in tumor homogenates in a model based on Walker-256 rats. Also, UT has been evaluated in terms of B16 melanoma lung metastases (8). Populations of CD4 and CD8a TLs and Th1/Th2 cytokines have been quantified in splenocyte cultures, but in healthy BALB/c mice (34). Our results indicate that there is a significant increase in the CD4/CD8a ratio of the TLs from the PBMCs of mice with melanoma treated with UT at a dose of 1000 mg/kg, in contrast to the TME. This increase implies the presence of a higher percentage of CD4+ TLs or helper cells, which are lymphocytes characterized by active participation in the cellular immune response because they are capable of activating NKs, NKT cells, macrophages, cytotoxic TLs, and B lymphocytes.
These results are similar to those of Domingues et al., who found upregulation of CD4+ TLs at doses 125 and 500 mg/kg of an hydroethanolic extract, although as mentioned above, they used healthy mice. Therefore, it is likely that a higher dose of UTPOA is necessary in mice with melanoma in order to achieve similar upregulation as in our study.
The activation level (CD44+) of the CD4+CD44+/CD8a+CD44+ ratio showed the opposite pattern, meaning that there was a significant increase at lower doses (50 and 500 mg/kg UT-POA), whereas at 1000 mg/kg, the ratio was lower but still greater than in the PC. These results suggest that UT-POA is capable of increasing not only the percentage of CD4+ TLs but also its activation level. It is possible that the activation level depends on the dose of UT-POA, but it was expected that this difference would remain in the TME. Nevertheless, it did not change significantly relative to PC. Although there are no differences in terms of percentages and activation of these cells in the TME, it is important to point out their presence, as well as the possibility of their activity’s being affected by tumor factors released by the melanoma. In this study, it was not possible to evaluate other systemic lymphocyte populations. Nevertheless, they were analyzed in the TME, where these lymphocyte populations (represented by total TLs, NKs, and NKT cells) were not affected by the UT-POA treatment. Their presence is also an important finding, as is the possible inhibitory effects of tumor factors.
The ability of mDCs to intervene and induce Th1 antitumor responses was demonstrated here.Our results show that UT-POA at 100 mg/kg induced a significant increase in the % of mDCs, and at this same concentration, the % of pDCs is 7.5 times lower than the percentage of mDCs. This finding could be explained by the studies of Zuniga et al., who reported that after a viral infection, pDCs can turn into mDCs via phenotypic and functional changes, including an improved ability to present antigens. In our case, it is possible that the UT-POA has an effect similar to that observed by Zuniga et al. Nevertheless, the mechanisms would have to be identified as well as whether these changed indeed occur or simply represent an effect directed specifically at mDCs while the pDCs are at normal levels.
TNF-α is produced not only by activated macrophages but also by tumor cells, and in cancer, this cytokine is reported to show paradoxical behavior because it is required for proliferation and function of NKs, TLs, BLs, macrophages, and DCs, and it is an important molecule to the cell-mediated elimination of certain tumors, at least during the acute stage of the disease. When TNF-α is produced by macrophages, it causes M1 polarization that could be useful at the acute stage. On the other hand, there is evidence that TNF-α is one of the main mediators of cancer-related inflammation and acts as a protumor factor, at least during the chronic stage of the disease (35). Our results show significant systemic upregulation of TNF-α at 1000 mg/kg UT-POA, and this increase correlates positively with the CD4/CD8a ratio, which in our model can be interpreted as a UT-POA-induced effect of an adaptive cellular response during the 29 days of treatment, which we can consider the acute stage. IL-17A also shows paradoxical behavior because it can impair the action of CD8+ TLs but can also induce inflammatory cytokines and chemokines that can attract CD8+ TLs, NKs, and DCs (31).
Our results revealed a negative correlation between IL-17A and the CD4/CD8a ratio at 1000 mg/kg UT-POA, which means that downregulation of IL-17A under the influence of UT-POA may be associated with a low systemic proportion of CD8a TLs. Although in this study, we could not assess the levels of IL-17A in the TME, it would useful to determine whether the presence of lymphocytes and DCs correlates with that cytokine. The evaluation of some immunological parameters allows us to conclude that UT-POA has better systemic effects than the effects in the TME, mainly because of the improved CD4/CD8a ratio and induction of Th1, inflammatory, or M1 macrophage polarization responses and a reduction in the Th17 response. Although these effects can be observed at high doses, we should take into account that after the first hydroalcoholic extraction, there was a second aqueous extraction, which may have dissolved some compounds and thus their potency may have been reduced. Nevertheless, UT exerted immunomodulatory effects. We recommend evaluating this extract after the first hydroalcoholic extraction. According to the above observation, the concentrations that we used should serve as a reference. Even though the model under study involves an aggressive type of cancer, our results are a good reference for future studies on the antitumor potential of UT-POA and its use as an adjunctive therapy during conventional treatment of cancer.
Acknowledgments: This work has been supported by EMINDES CANCER SAC (a company engaged in cancer-related research and development). We would like to thank the company Becton Dickinson del Uruguay SA, Peru Branch, for their assistance with flow cytometry. We would also like to thank the members of the Immunology Lab (LID-108) and the Research and Development Labs (LID), Cayetano Heredia Peruvian University, Lima-Peru.
Author Contributions: ILR and CN participated in the conception and design of the study protocol and in procurement of funding. ILR, YA, and LK participated in the collection of samples, experiments, and statistical analysis. ILR, CN, YA, LK, and JA participated in the data analysis and writing of the manuscript. All the coauthors critically reviewed the manuscript.
Funding Sources: this study was funded by EMINDES SAC.
Conflicts of Interest: there are no potential conflicts of interest.
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002 . CA Cancer J Clin. 2005;55(2):74–108. [Enlace]
2. Naldi L, Altieri A, Imberti GL, Giordano L, Gallus S, La Vecchia C, et al. Cutaneous malignant melanoma in women. Phenotypic characteristics, sun exposure, and hormonal factors: A case-control study from Italy . Ann Epidemiol. 2005;15(16):545–50. [Enlace]
3. Dwek M, Brooks SA, Schumacher U. NY: Metastasis Research Protocols. Humana Press; 2012.
4. Park WB, Lyu SY, Kim JH, Choi SH, Chung HK, Ahn SH, et al. Inhibition of tumor growth and metastasis by Korean mistletoe lectin is associated with apoptosis and antiangiogenesis. Cancer Biother Radiopharm. 2001;16(5):439–47. [Enlace]
5. Currier NL, Miller SC. Echinacea purpurea and melatonin augment natural-killer cells in leukemic mice and prolong life span . J Altern Complement Med. 2001;7(3):241–51. [Enlace]
6. Liu JD, Chen SH, Lin CL, Tsai SH, Liang YC. Inhibition of melanoma growth and metastasis by combination with (-)-epigallocatechin-3-gallate and dacarbazine in mice . J Cell Biochem. 2001;83(4):631–42. [Enlace]
7. Aguilar L, Rojas P, Marcelo A, Plaza A, Bauer R, Reininger E , et al. Anti-inflammatory activity of two different extracts of Uncaria tomentosa (Rubiaceae) . J Ethnopharmacol. 2002;81(2):271–6. [Enlace]
8. Fazio AL, Ballén D, Cesari IM, Abad MJ, Arsenak M, Taylor P. An ethanolic extract of Uncaria tomentosa reduces inflammation and B16- BL6 melanoma growth in C57BL/6 mice. Bol Latinoam Caribe Plant Med Aromaticas. 2008;7:217–24. [Enlace]
9. Dreifuss AA, Bastos-pereira AL, Avila TV, Soley Bda S , Rivero AJ , Aguilar JL , et al. Antitumoral and antioxidant effects of a hydroalcoholic extract of cat’s claw (Uncaria tomentosa) (Willd. Ex Roem. & Schult) in an in vivo carcinosarcoma model . J Ethnopharmacol. 2010;130(1):127-33. doi: 10.1016/j.jep.2010.04.029. [Enlace]
10. García Giménez D, García Prado E, Sáenz Rodríguez T, Fernández Arche A, De La Puerta R. Cytotoxic effect of the pentacyclic oxindole alkaloid mitraphylline isolated from Uncaria tomentosa bark on human Ewing’s sarcoma and breast cancer cell lines . Planta Med. 2010;76(2):133-6. doi: 10.1055/s-0029-1186048. [Enlace]
11. de Paula CL, Fonseca F, Perazzo F, Cruz FM, Cubero D, Trufelli DC, et al. Uncaria tomentosa (cat’s claw) improves quality of life in patients with advanced solid tumors . J Altern Complement Med. 2015;21(1):22-30. doi: 10.1089/acm.2014.0127. [Enlace]
12. Santos Araújo Mdo C, Farias IL, Gutierres J, Dalmora SL, Flores N, Farias J, et al. Uncaria tomentosa-adjuvant treatment for breast cancer: clinical trial . Evid Based Complement Alternat Med. 2012;2012:676984. doi: 10.1155/2012/676984. [Enlace]
13. Falkiewicz B. Vilcacora [Uncaria tomentosa (Willd.) DC. and Uncaria guianensis (Aublet) Gmell.]–a review of published scientific literature . Case Rep Clin Pr Rev. 2001;2(4):305–16. [Enlace]
14. Sheng Y, Pero RW, Wagner H. Treatment of chemotherapy-induced leukopenia in a rat model with aqueous extract from Uncaria tomentosa. Phytomedicine. 2000;7(2):137–43. [Enlace]
15. Sheng Y, Bryngelsson C, Pero RW. Enhanced DNA repair, immune function and reduced toxicity of C-MED-100, a novel aqueous extract from Uncaria tomentosa . J Ethnopharmacol. 2000;69(2):115–26. [Enlace]
16. Aquino R, De Feo V, De Simone F, Pizza C, Cirino G. Plant metabolites. New compounds and anti-inflammatory activity of Uncaria tomentosa . J Nat Prod. 1991;54(2):453–9. [Enlace]
17. Sandoval M, Charbonnet RM, Okuhama NN, Roberts J, Krenova Z, Trentacosti AM, et al. Cat’s claw inhibits TNFalpha production and scavenges free radicals: role in cytoprotection . Free Radic Biol Med. 2000;29(1):71–8. [Enlace]
18. Rakoff-nahoum S. Why Cancer and Inflammation ? Yale J Biol Med. 2006;79(3-4):123–30. [Enlace]
19. Coussens LM, Werb Z. Inflammation and cancer . Nature. 2002;420(6917):860–7. [Enlace]
20. Zhang S, Li W, Xia Z, Mao Y. CD4 T cell dependent tumor immunity stimulated by dendritic cell based vaccine . Biochem Biophys Res Commun. 2011;413(2):294-8. doi: 10.1016/j.bbrc.2011.08.089. [Enlace]
21. Schmidt W, Schweighoffer T, Herbst E, Maass G, Berger M, Schilcher F, et al. Cancer vaccines: the interleukin 2 dosage effect . Proc Natl Acad Sci USA. 1995;92(10):4711–4. [Enlace]
22. Allam M, Julien N, Zacharie B, Penney C, Gagnon L. Enhancement of Th1 type cytokine production and primary T cell activation by PBI-1393 . Clin Immunol. 2007;125:318–27. [Enlace]
23. Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells . Cell Res. 2010;20(1):4-12. doi: 10.1038/cr.2009.138. [Enlace]
24. Coffman RL. Origins of the T(H)1-T(H)2 model : a personal perspective . Nat Immunol. 2006;7(6):539–41. [Enlace]
25. Goto S, Sato M, Kaneko R, Itoh M, Sato S, Takeuchi S. Analysis of Th1 and Th2 cytokine production by peripheral blood mononuclear cells as a parameter of immunological dysfunction in advanced cancer patients . Cancer Immunol Immunother. 1999;48:435–42. [Enlace]
26. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease . Altern Med Rev. 2003 Aug;8(3):223-46. [Enlace]
27. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy . Nat Rev Cancer 2004;4(1):11–22. [Enlace]
28. Lee AF, Sieling PA, Lee DJ. Immune correlates of melanoma survival in adoptive cell therapy . Oncoimmunology. 2013;2(2):e22889. [Enlace]
29. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages . Nat Immunol. 2005;6(11):1123–32. [Enlace]
30. Steiner GE, Newman ME, Paikl D, Stix U, Memaran-Dagda N, Lee C, et al. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate . Prostate. 2003;56(3):171–82. [Enlace]
31. Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy . Nat Rev Immunol. 2010;10(4):248–56. doi: 10.1038/nri2742. [Enlace]
32. Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection . Nat Immunol. 2004;5(12):1227–34. [Enlace]
33. Aspord C, Leccia MT, Charles J, Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL . Cancer Immunol Res. 2013;1(6):402–15. doi: 10.1158/2326-6066.CIR-13-0114-T. [Enlace]
34. Domingues A, Sartori A, Maria L, Valente M, Golim MA, Siani AC, et al. Uncaria tomentosa aqueous-ethanol extract triggers an immunomodulation toward a Th2 cytokine profile . Phyther Res. 2011;25(8):1229–35. doi: 10.1002/ptr.3549. [Enlace]
35. Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion . Br J Cancer. 2010;102(4):639–44. doi: 10.1038/sj.bjc.6605530. [Enlace]
Address: Av. Honorio Delgado 430 Urb. Ingeniería – San Martin de Porres. Lima, Peru.
Phone number: 511-998674601
E-mail: ivan.lozada@upch.pe
Received: 3/5/2015
Approved: 7/23/15