Original Article
Immunomodulation of Uncaria tomentosa over dendritic cells, IL-12 and profile TH1/TH2/TH17 in breast cancer.
César Núñez1,2,a, Iván Lozada-Requena1,2,b, Tíndara Ysmodes1,c, Daniel Zegarra1,d, Fatima Saldaña1,d, José Aguilar1,e
1 Immunology laboratory. Deparment of cellular and molecular sciences. Faculty of sciences an philosophy. Universidad Peruana Cayetano Heredia. Lima, Peru.
2 EMINDES SAC (Empresa de Investigación y Desarrollo en Cáncer). Lima, Peru.
a Physician; b master’s
degree in science; c licentiate in
science; d degree of bachelor in science; e rheumatologist
specializing in immunology
ABSTRACT
Objetives. This study aimed to research the in vitro immunomodulatory effects of an Uncaria tomentosa hydroalcoholic extract standardized (5.03%, pentacyclic oxindole alkaloids) (UT-POA) on the immunophenotype of dendritic cells (DC) subsets, Th1, Th2, Th17 and IL-12 cytokines from patients with stage II breast cancer (BCII) and healthy women (H). Materials and methods. Blood of 11 H and 7 BCII was obtained, PBMC were isolated and cultured for 2h with/without various concentrations of UT-POA and stimulated or not with LPS for 24h. PBMC were labeled with specific antibodies for DC and in the supernatant we measured Th1/Th2/Th17 cytokines, both by flow cytometry. Furthermore IL-12 was measured by ELISA. Results. UT-POA did not alter DC or accessory molecules expression in BCII. However, H exhibited a decrease in the percentage of mDC (myeloid DC) and an increase in HLA-DR and CD86 expression at 1000 μg/ mL. IL-12 secretion was modified only in the H group, increasing p70 subunit and decreasing p40 subunit. UT-POA increased Th1 (IFN-γ and IL-2), Th2 (IL-4) and Th17 (IL-17) secretion in both groups. Conclusions. UT-POA increased the production of cytokines related with anti-tumoral response at concentrations of 500-1000 μg/mL. This positive effect should be evaluated not only systemically but also in the tumor microenvironment in further studies. UT-POA may be a useful phytochemical in chemoprevention and in the alternative use in cancer therapies.
INTRODUCTION
Uncaria tomentosa (Willd.) DC. (Rubiacaceae) (UT) or cat’s claw is a vine that grows in the
Peruvian jungles and in tropical areas of South and Central America. Indigenous
groups such as the Asháninkas used this plant to
improve their health (1). UT is used as a contraceptive, an
anticancer drug, and to treat inflammatory and gastrointestinal disorders. It is consumed as tea (3). Sandoval- Chacón et al. and Aguilar et al. Demonstrated its
anti- inflammatory activity in vitro (4 ,5). Aquino et al. did the same in vivo in murine inflammation models (2). Dreifuss et al. showed its antitumor and antioxidant effects in vivo in a rat model of carcinoma (6 ,7).
The role of chronic inflammation during the development and progression of a tumor is known; therefore, the link between inflammation and cancer is not surprising (8,9). UT has been shown to inhibit the translocation of NF-κB (4 ,5), a factor that activates genes involved in the anti-inflammatory response and in apoptosis regulation. In healthy cells, activation of NF-κB promotes apoptosis, but in malignant cells, it promotes survival, whereas inhibition of its activity may reduce tumor growth (10). Allen-Hall L et al. showed that treatment with UT inhibits lipopolysaccharide (LPS)-dependent activation of specific components of the AP-1 signaling pathway (11). An effective antitumor response requires active participation of antigen-presenting cells (APCs) responsible for presentation of tumor antigens (12). APCs are important because defects in the function of lymphocytes that infiltrate the tumor in a patient with cancer (and T lymphocytes [TLs] of mice with tumors) can be completely reversed when an effective antigen presentation takes place and these lymphocytes are provided with exogenous IL-2. Dendritic cells (DCs) are the most powerful APCs (13,14). DCs play a central role in antitumor immunity because they process tumor antigens and stimulate antigen-specific TLs (15,16). An optimal costimulatory signal between the two cell types is necessary for effective presentation of antigens (12). A population of isolated DCs from peripheral blood of patients with breast or head and neck cancer shows a reduced ability to group and stimulate allogeneic antigen specific responses by TLs. Isolated DCs of these patients show a lower level of MHC II (HLA-DR) expression and of DC costimulatory molecules in comparison with healthy donors (17). Our team has demonstrated that a hydroalcoholic UT extract reduces the proportion ( %) of myeloid DCs ( mDCs) and upregulates HLADR and CD86 in a dose-dependent manner in the peripheral blood mononuclear cells (PBMCs) of patients with rheumatoid arthritis, thus exerting an immunomodulatory effect (18).
The new paradigm of the
immune balance not only involves CD8+ cytotoxic TLs, CD4+ TLs subsets,
regulatory lymphocytes, γδ lymphocytes, natural killer cells, natural
killer T cells, macrophages, DCs, and other innate-immunity cells but also proinflammatory cytokines and Th1, Th2, Th17, and immunoregulatory cytokines. IL-12 favors the Th1 profile,
and Th1 is one of the most effective responses against tumors. Macrophages and DCs participate in
the antitumor response by producing IL-12, among other functions inherent in
innate immunity. In an in vitro study, we found that UT increases
expression of IL-12p70 in PBMCs of healthy subjects (unpublished data); this
result suggests that UT can promote a Th1 response. Domingues
A. et al. demonstrated in healthy BALB/c mice that UT promotes the Th2
profile (IL-4, IL-5)
in a dose-dependent manner but inhibits the Th1 profile (IL-2, TNF-α, IFN-k) (19).
Fazio A.L. et al. showed that in the peritoneal macrophages of C57/BL6
mice with B16/BL6 melanoma, UT downregulates IL-6 and NO but not TNF-α (20). Urdanibia et al.
uncovered an antiinflammatory and antitumor effect of another species
of the genus Uncaria, U. guianensis,
when they observed inhibition of the growth of a breast tumor (4T1); this
effect is probably related
to downregulation of inflammatory mediators such as TNF-α and IL-6 via inhibition of the NF-κB pathway (21). In
the present study, we report in vitro antitumor activity of a UT hydroalcoholic extract as well as an immunomodulatory
effect on DCs and on IL12 and on Th1, Th2, and Th17
cytokines according to analysis of human PBMCs.
MATERIALS AND METHODS
HUMAN SUBJECTS
The participants were 10 healthy women (group H) with mammograms negative for breast adenocarcinoma (age 46.6 ± 9.1 years) and seven women with a histological diagnosis of stage II breast adenocarcinoma (age 54.7 ± 12.7 years; group BCII). The inclusion criteria were as follows: no history of treatment with any chemo-or radiotherapy or surgical intervention, no history of any other type of cancer, immunodeficiencies, and/or autoimmune diseases; no cytostatic drugs in the last 12 months, and no chronic diseases. Peripheral-blood samples were collected via venipuncture (36 ml).
HYDROALCOHOLIC UT EXTRACT STANDARDIZED AT 5.03% PENTACYCLIC OXINDOLE ALKALOIDS (UT-POA)
The extract was provided by Peruvian Heritage SAC. A hydroalcoholic
extract was prepared
ISOLATION of PBMCs
These cells were isolated by density gradient centrifugation with 1.077 g/mL Histopaque (Sigma, St. Louis, MO, USA). Beforehand, tubes had been prepared with 10 mL of Histopaque. The blood samples were diluted 1:1 with complete RPMI 1640 (Sigma). The diluted blood (30 mL) was added onto the Histopaque and centrifuged at 400 × g for 30 minutes. The PBMCs were collected and stored on ice.
CELL CULTURE AND TEST GROUPS
The PBMCs were cultured in the RPMI 1640 medium supplemented with 10%
bovine fetal serum ( Hyclone), 100 U/mL penicillin and
100 μg/mL streptomycin (Sigma, St Louis, MO, USA). We
placed 106
PBMCs in cytometry tubes and incubated them at 37°C and 5% CO2 for 2
hours with/without UT-POA. E. coli lipopolysaccharides were either added
or not added (Sigma, St Louis, MO, USA) (LPS 1 µg/mL), standardized in our lab,
and the cells were incubated at 37°C and 5% CO2 for 24 hours. The test
groups for BCII and H patients were as follows: A) Basal = PBMCs plus UT-POA
vehicle; B) LPS = PBMCs plus vehicle and stimulated with LPS (1 µg/mL); C) UG50
= PBMCs plus UT-POA (50 µg/mL) and stimulated with LPS; D) UG500 = PBMCs plus
UT-POA (500 µg/mL) and stimulated with LPS; and E) UG1000 = PBMC plus UTPOA
(1000 µg/mL) and stimulated with LPS.
FLOW CYTOMETRIC ANALYSIS OF DCs AND HLADR/CD86
Prior to the flow cytometry, the culture supernatants were collected for quantification of the cytokines, and the cells were washed and resuspended in a cell wash solution (1% fetal bovine serum in PBS [pH 7.4]). The cells were immediately labeled with specific antibodies: anti-Lin1–FITC, anti-HLA-DR– PerCP, anti-CD11c–APC/antiCD123–APC, and anti-CD86–PE (Becton Dickinson, San Jose, CA, USA). The labeled cells were incubated for 30 minutes at 2–8°C and washed twice with 1 mL of the cell wash solution with centrifugation at 400 × g for 5 minutes.
The cells were resuspended in 500 μL of
the cell fixative (1% paraformaldehyde in PBS, pH 7.4) and stored at 4°C until
analysis on a FACSCantoTM II flow cytometer (BD Immunocytometry
Systems, USA). The data were analyzed in the Summit 4.3
software ( Dako Colorado, Inc., USA). The percentages
of myeloid DCs ( mDCs; CD11c+HLADR+Lin-1 -) and plasmacytoid dendritic
cells ( pDCs; CD123+HLA-DR+Lin-1-) were determined.
The mean intensity of
fluorescence (MIF) of HLA-DR and CD86 was measured in
each subset.
QUANTIFICATION OF TH1, TH2, and TH17 CYTOKINES BY MEANS OF A CYTOMETRIC BEAD ARRAY (CBA)
A CBA kit was used to quantify the Th1, Th2, and Th17 cytokines (BD,
San Jose CA, USA) in 50 μL of the cell culture supernatant. The following proteins were analyzed:
interferon γ (IFN-γ), interleukin 2 (IL-2), tumor necrosis factor α (TNF-α), IL-4, IL-6, IL-10, and IL-17A. The
manufacturer’s recommendations were followed strictly.
The data were acquired on a FACSCanto™ II flow cytometer (BD Immunocytometry
Systems, USA) and analyzed in the BD™ Cytometric Bead Array Software, version
1.4.
MEASUREMENT OF IL-12
AND IL-6 LEVELS BY AN ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA) We used ELISA kits ( EIAOpt BDBiosciences, San Diego, CA, USA) for quantification of
IL-12p40, IL-12p70, and IL-6. The manufacturer’s recommendations were followed strictly. The IL-6 levels that were determined by CBA were above the upper boundary of the
detection limit (5000 pg/mL); therefore, it was
necessary to dilute the samples to analyze them with an ELISA.
STATISTICAL ANALYSIS
The results are expressed as mean ± standard error of the mean. Kruskal-Wallis nonparametric tests were performed and followed by Dunn’s multiple-comparison test and the t test followed by the Mann-Whitney (two tailed) rank-sum test in the GraphPad Prism software, version 6.00 for Mac ( GraphPad Software, La Jolla, CA, USA). Differences with p < 0.05 were considered statistically significant.
ETHICAL CONSIDERATIONS
The Human Ethics Institutional Committee of the Cayetano Heredia Peruvian University approved this study’s protocol (registration code 57825), and each participant signed an informed consent form.
RESULTS
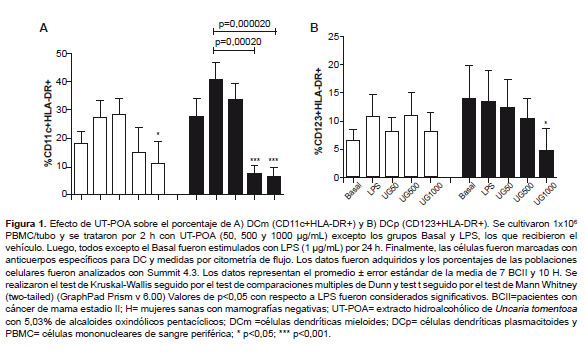
mDCs, pDCs, and HLA-DR/CD86
The quantification of mDCs,
pDCs, and of expression of HLA-DR and CD86 was performed on 106 PBMCs. After treatment with
UT-POA, a dose-dependent decrease in % of mDCs in group H was observed; this effect was significant at
doses 500 and 1000 µg/mL (p < 0.05; Figure 1). Group BCII showed a
significant decrease in % of mDCs at 1000 μg/mL UT-POA (p < 0.05;
Figure 1A). UT-POA
significantly decreased % of pDCs
at 1000 μg/mL (p < 0.05) in group H (Figure 1B).
The HLA-DR molecule in mDCs
and pDCs was upregulated significantly (p < 0.05)
in group H at the UT-POA dose 1000 μg/mL, whereas no change was detected in group
BCII (Figure 2A and 2C). No changes in CD86 expression were
observed (Figure 2B and 2D).
IL-12p70 was upregulated significantly in group BCII in response to 1000 μg/mL
UT-POA (p < 0.05); however, it increased in a dose-dependent manner in group
H (significantly in response to doses 500 and 1000 μg/mL;
p < 0.05; Figure 3A). In both groups, IL-12p40 showed dosedependent
downregulation, significant only in group H at the
UT-POA dose 1000 μg/mL in comparison with LPS (p < 0.05; Figure 3B).
In groups BCII and H,
IFN-γ and IL-2 levels increased
in a dose-dependent manner at UT-POA doses 500 and 1000 μg/mL
(p < 0.05) as compared to LPS treatment. Higher levels of IFN-γ and IL-2 were observed in
group H than in group BCII (Figure 4A and 4B). The levels of TNFα did
not change in any group (Figure 4C).
Th2 CYTOKINES
There was a dose-dependent increase in IL-4
levels in groups BCII and H (p < 0.05) at UT-POA doses 500 and 1000 μg/mL
as compared to LPS treatment. We also found that group H produced more IL-4
than group BCII did (Figure 4D). IL-6 and IL-10 levels did not change (Figure 4E and 4F). In contrast, IL-6 levels decreased significantly at the UT-POA dose
50 μg/mL in group H; these IL-6
concentrations later remained at the same level as those in the LPS group
(Figure 4F). There was a dose-dependent trend toward an increase in IL-10
levels, with a higher concentration of this cytokine observed in group BCII (Figure 4E).
There was a dose-dependent increase in IL-17 levels in groups BCII and H, and this effect became statistically significant at the UT-POA dose 1000 µg/mL (p < 0.05; Figure 4G)
DISCUSSION
UT is an immunomodulatory, anti-inflammatory and antioxidant phytochemical. In other studies, researchers found correlations of the concentration of pentacyclic oxindole alkaloids (POAs) in its leaves, bark, and roots with the immunomodulatory properties of this plant (22). Several phytochemicals that were prepared from the ethanolic extracts of the bark and from alkaloid-rich parts of UT have been standardized by their oxindole alkaloid content (1,23). Nevertheless, this situation does not mean that the POAs are the cause of UT’s effects because there are products such as C-Med 100®—a water-soluble and ultra-filtered UT patented extract (for removal of high molecular-weight conjugates; >10 kDa)—which contains carboxy alkyl esters (CAEs) as active ingredients (8–10%) and is almost free of oxindole alkaloids (≤0.05%). Yet this product has immunomodulatory effects (24). Therefore, it is possible that various active ingredients of this plant work in synergy.
We tested whether UT is capable of modifying the immunophenotype of DCs and can influence production of IL12 and Th1, Th2, and Th17 cytokines in vitro by human PBMCs. We found that UT-POA apparently has opposite immunomodulatory effects on DCs and on their HLA-DR marker: in the case of DCs, UT-POA upregulates these cell-specific markers; as for HLA-DR, UT-POA increases the expression of this maturity-related molecule. UT-POA did not induce activation of these cells because it has no effect on the costimulatory molecule CD86. There are few studies on the effects of UT on human DCs. Kim K.S. et al. tested other species such as Uncaria rhynchophylla, and in particular uncarinic acid (URC), and found that the DCs isolated from healthy human PBMCs increase the expression of CD1A, CD38, CD40, CD54, CD80, CD83, CD86, HLA-DR, and DC-Lamp after treatment with URC in the range 0.001 to 10 μM (25). In the case of UT, Nunez C. et al. and Lozada-Requena et al. showed that the proportion (%) of mDCs among PBMCs of patients with rheumatoid arthritis and in healthy subjects decreases in a dose-dependent manner, whereas HLA-DR and CD86 are upregulated in patients with arthritis but undergo no changes in healthy subjects (18,26). We confirmed the findings of Lozada-Requena et al. regarding the decrease in % of mDCs in group H, and we observed the same result in group BCII. Nevertheless, in the case of % of pDCs, our results were the opposite because we actually observed downregulation of this subset in group H. It is possible that these patients’ disease has a protective/blocking effect toward UT-POA, via typical evasion mechanisms of tumors and via factors released by tumors (or some other unknown mechanism). Accordingly, we did not detect any immunomodulatory effect of UT-POA on the pDCs and HLA-DR/CD86 cells of patients with breast cancer, except for a decrease in the number of mDCs at high doses. The systemic downregulation of this mDC subset should not be necessarily viewed as a bad prognosis. This is because DCs represent 0.1–1.0% of blood immunocompetent cells, and migration of DCs to the tumor microenvironment is possible. Additional studies are needed with standardized extracts at different doses and in the tumor microenvironment.
The production of
IL-12p70 increased while that of IL-12p40 decreased. IL-12p70 is responsible
for the DC-mediated Th1 polarization. Our findings show that PBMCs that are stimulated in vitro with
UT-POA produce greater amounts of cytokines of the types Th1 (IFN‐γ and IL‐2), Th2 (IL-4), and Th17 (IL-17) in groups BCII and H. Although these
effects are observed at high doses, we must take into
account that the 50% inhibitory concentration (IC50)
of UT for human PBMCs is 16 mg/ mL, which indicates that UT does not affect
cellular viability of these PBMCs (18). We propose measuring
metabolic viability of these cells in terms of UT. It should
also be noted that during the preparation of UT, there are filtering
steps that may dilute the extract; therefore, the concentrations that we used
can serve as a reference.
We demonstrated that
expression of neither IL-12p70 nor the IL-12p40 subunit was changed by UT-POA in group BCII. In contrast, IL-12p70 was upregulated and
IL-12p40 was downregulated in group H. A possible
explanation is that a part of IL-12p40 dimerizes with IL-12p35 to form
IL-12p70. It is well known that the IL-12 family
consists of IL-12, IL-23, and IL-27, and that IL-12p40 not only generates IL-12
but also forms IL-23 by joining with IL12p19. Therefore, it is possible that a
part of IL-12p40, which was downregulated in group H,
dimerized with the subunit IL-12p19 (27.28). This hypothesis should be tested by measuring IL-23. According to our
findings, at least in healthy subjects, UT-POA promotes production of IL-12.
Patients (group BCII) may have a PBMC disorder that inhibits a favorable
response to treatment with the UTPOA extract. The reason for this lack of
response is the same as the reason we proposed for DCs, that is, tumor evasion
or other unknown mechanisms.
Regarding the
Th1/Th2/Th17 profile, we found that UT POA increased expression of cytokines
of the types Th1 (IFN-γ and IL-2), Th2 (IL-4), and
Th17 (IL17A) in groups BCII and H. There are few studies on the effects of UT
on Th1, Th2, and Th17 cytokines. Domingues A. et
al. reported that the splenocytes of mice treated
with a hydroalcoholic cat’s claw extract have a Th2 profile
characterized by an increase of IL-4 and IL-5 levels at the dose 500 µg/mL,
whereas the same concentration produced a reduction in concentrations of Th1 cytokines (IFN-γ, IL-2, and TNF-α) (19).
Fazio A.L. et al. found that a Th1 response (TNF-γ) and Th2 response (IL-6) were reduced in the supernatant of peritoneal macrophage
cultures treated with UT after LPS stimulation. Those authors also demonstrated
that this reduction correlates with a deficiency in the NF-κB pathway (20).
Although those studies were performed on animal models, we demonstrated that
cat’s claw can have different effects on the expression of different cytokines,
and that the results may not be extrapolated from mice to humans. We showed
induction of a Th1 response in group H, probably due
to the IL-12p70 downregulation, and this change can therefore explain the
upregulation of IFN-γ and IL-2. It is possible,
however, that in group BCII, UTPOA directly
stimulates expression of IFN-γ and IL-2 via mechanisms that need to be
researched further. Both cytokines are important for the immune response,
whereas IL-2 works as a growth factor necessary for the survival and
differentiation of TLs and is produced mainly by CD4+
TLs. This observation can explain why the presence or upregulation of IL-2 can
improve an adaptive antitumor response. IFN-γ performs a direct function in antitumor
immunity, not only in adaptive immunity but also in innate immunity, because
this cytokine can have different effects on endothelial and stromal components
in the tumor microenvironment. These effects may induce chemokines that attract
effector cells from both arms of the immune system and downregulate
angiogenesis and adhesion molecules of endothelial cells.
As for TNF-α, inhibition of its
production by UT is well known. Various studies have shown an anti-inflammatory effect of UT in a variety of in vitro and
in vivo models. Our results do not show TNF-α changes
in the test groups. It is possible that unknown mechanisms prevent its
inhibition or that our duration of treatment with UT-POA and duration of the LPS stimulation were
not sufficient, and that these variables should be
standardized for this particular model. Furthermore, the other studies were performed on standardized cell lines or on specific
cell lines such as peritoneal macrophages. In contrast, we used PBMCs, which
produce other leukocytes, which may cause greater variability in the
measurements.
UT-POA increased the
IL-4 levels in both groups. TLs, mast cells, and other cell populations are the
source of IL-4, and it is required for Th2 differentiation. The use of PBMCs
implies that the main source of IL-4 is TLs. Although a reduced Th2 profile can
be expected due to a prevalent Th1 profile, UT-POA also enhanced the Th2 (IL-4)
profile, showing once again its immunomodulatory effect. Despite the weak
relevance of IL-4 to antitumor immunity, because this cytokine can induce
polarization of M2 or TAM (tumor-associated macrophages) that are more protumor, IL-4 is expected to collaborate with B
lymphocytes that produce IgG and IgE, and thereby to
increase the expression of MHC. Therefore, these effects should also improve
activation of TLs and the function of eosinophils (8).
Some antibodies against molecules present
in cancer cells may inhibit oncogenic signals and promote tumor destruction by
binding to Fc receptors of macrophages, granulocytes, and NK cells. Antibodies
may also promote presentation of tumor antigens by APCs via formation of an
immune complex (8). For these reasons, the role Th2 (IL-4) differentiation
in the antitumor response should not be ignored.
We showed that IL-10 was not affected by UT-POA in either group BCII or group H. In contrast, production of IL-6 decreased at UT-POA dose 50 µg/mL initially, and then in a dose-dependent manner, it reached the levels of the LPS group. Lemaire et al. demonstrated an inhibitory effect of two UT aqueous extracts on IL-6 in rat alveolar macrophages (29), but here, we demonstrated that UT-POA can have different effects at different concentrations. These findings underscore the importance of determining the optimal concentration at which these effects occur.
Th17 lymphocytes were recently identified in humans and are reported to play a proinflammatory role in autoimmune diseases. Th17 lymphocytes are also necessary for the antitumor response because they induce production of chemokines by primary tumors, which attract DCs, TLs, and NK cells to the tumor microenvironment (30). On the other hand, there is also evidence of a correlation between regulatory TLs and LTh17 in patients with invasive breast cancer, suggesting that upregulation of these subsets is the result of conditions that favor aggressiveness of the tumor and poor prognosis. In other tumors, such as ovarian tumors, Th17 cells induce Th1-type chemokines and the recruitment of effector cells. Thus, Th17 cells and regulatory TLs can play a dual role in the tumor microenvironment (31). These apparent discrepancies involving Th17 and IL-17A should be elucidated as soon as possible. Currently there are no studies that assess the effects of UT-POA on the expression of IL-17. Our results show that there is a dose-dependent increase in the production of IL-17A in groups H and BCII. The production of IL-17A was found to be always higher in group H than in group BCII, suggesting that although breast cancer reduces the secretion of IL-17A, this process is restored by UT-POA.
The data obtained in the PBMC experiments involving patients with breast cancer point to a clinically important immunomodulatory effect of cat’s claw on the expression of cytokines of the types Th1 (IFN-γ and IL-2), Th2 (IL-4), and Th17 (IL-17A). This effect needs further research to confirm the cytoprotective activity of this phytochemical during an antitumor response.
Acknowledgments: This work has been supported by EMINDES CANCER
SAC (a company engaged in cancer related research and development). The flow cytometry
service was provided by the Immunology Lab (LID-108)
and by the Research and Development Lab (LID), Cayetano
Heredia Peruvian University, Lima-Peru.
Author contributions: CN and ILR participated in the conception and
design of the study protocol and in procurement of funding. ILR and TY
participated in the collection of samples, experiments, and statistical
analysis. CN, ILR, TY, DZ, FS, and JA participated in the data analysis and
writing of the manuscript. All the coauthors critically reviewed the
manuscript.
Funding Sources: this study was funded by
EMINDES SAC.
Conflicts of Interest: There are no potential conflicts of interest.
References
1. Keplinger K, Laus G, Wurm M, Dierich MP, Teppner H. Uncaria tomentosa (Willd.) DC.—ethnomedicinal use and new pharmacological, toxicological and botanical results . J Ethnopharmacol. 1999;64(1):23-34.
2. Aquino R, De Feo V, De Simone F, Pizza C, Cirino G. Plant metabolites. New compounds and anti-inflammatory activity of Uncaria tomentosa. J Nat Prod. 1991;54(2):453-9.
3. Pilarski R, Poczekaj-Kostrzewska M, Ciesiolka D, Szyfter K, Gulewicz K. Antiproliferative activity of various Uncaria tomentosa preparations on HL-60 promyelocytic leukemia cells . Pharmacol Rep. 2007; 59(5):565-72.
4. Sandoval-Chacón M, Thompson JH, Zhang XJ, Liu X, Mannick EE, Sadowska-Krowicka H, et al. Antiinflammatory actions of cat’s claw: the role of NF-kappaB. Aliment Pharmacol Ther. 1998; 12(12):1279-89.
5. Aguilar JL, Rojas P, Marcelo A, Plaza A, Bauer R, Reininger E, et al. Anti-inflammatory activity of two different extracts of Uncaria tomentosa (Rubiaceae) . J Ethnopharmacol 2002; 81(2):271-6.
6. Dreifuss AA, Bastos-Pereira AL, Ávila TV, Soley Bda S, Rivero AJ, Aguilar JL, et al. Antitumoral and antioxidant effects of a hydroalcoholic extract of cat’s claw (Uncaria tomentosa) (Willd. Ex Roem. & Schult) in an in vivo carcinosarcoma model . J Ethnopharmacol. 2010; 130(1):127-33. doi: 10.1016/j.jep.2010.04.029.
7. Dreifuss AA, Bastos-Pereira AL, Fabossi IA, Livero FA, Stolf AM, Alves de Souza CE, et al. Uncaria tomentosa exerts extensive anti-neoplastic effects against the Walker-256 tumour by modulating oxidative stress and not by alkaloid activity . PLoS One. 2013; 8(2):e54618. doi:10.1371/journal.pone.0054618.
8. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy . Nat Rev Cancer . 2004;4(1):11-22.
9. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24-37.
10. Karin M. NF-kappaB as a critical cink between inflammation and cancer . Cold Spring Harb Perspect Biol. 2009; 1(15):a000141. doi: 10.1101/cshperspect.a000141.
11. Allen-Hall L, Arnasond JT, Cano P, Lafrenie RM. Uncaria tomentosa acts as a potent TNF-alpha inhibitor through NF-kappaB . J Ethnopharmacol. 2010;127(3):685-93. doi: 10.1016/j.jep.2009.12.004.
12. Fuertes MB, Zwirner NW. Estrategias celulares de defensa contra cáncer. In: Aguilar JL, editor. Bases de la Inmunología Clínica. 1ra ed. Lima: Sociedad Peruana de Inmunología (SPI); 2013. p.147-68.
13. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells . Annu Rev Immunol. 2003;21:685-711.
14. Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo . Proc Natl Acad Sci USA. 1999;96(3):1036-41.
15. Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differ entiation in cancer . Clin Cancer Res. 2000;6(5):1755-66.
16. Esche C, Lokshin A, Shurin GV, Gastman BR, Rabinowich H, Watkins SC, et al. Tumor’s other immune targets: dendritic cells . J Leukoc Biol. 1999; 66(2):336–344.
17. Tas M, Simons P, Balm F, and Drexhage H. Depressed monocyte polarization and clustering of dendritic cells in patients with head and neck cancer: in vitro restoration of this immunosuppression by thymic hormones . Cancer Immunol Immunother. 1993;36(2):108-14.
18. Núñez C, Lozada-Requena I, Akamine I, Carbajal L, Aguilar JL. Efecto de Uncaria tomentosa (Uña de Gato) sobre la población y activación de células dendríticas de sangre periférica de pacientes con artritis reumatoidea . Acta Med Per. 2008;25(3):135-9.
19. Domingues A, Sartori A, Valente LM, Golim MA, Siani AC, Viero RM. Uncaria tomentosa aqueous-ethanol extract triggers an immunomodulation toward a Th2 cytokine profile . Phytother Res. 2011;25(8):1229-35. doi: 10.1002/ptr.3549.
20. Fazio AL, Ballen D, Cesari IM, Abad MJ, Arsenak M, Taylor P. An ethanolic extract of Uncaria tomentosa reduces inflammation and B16-BL6 melanoma growth in C57BL/6 mice . Bol Latinoam Caribe Plant Med Aromaticas. 2008; 7(4):217-224.
21. Urdanibia I, Michelangeli F, Ruiz MC, Milano B, Taylor P. Anti-inflammatory and antitumoural effects of Uncaria guianensis bark . J Ethnopharmacol. 2013;150(3):1154-62. doi: 10.1016/j.jep.2013.10.055.
22. Winkler C, Wirleitner B, Schroecksnadel K, Schennach H, Mur E, Fuchs D. In vitro Effects of Two Extracts and Two Pure Alkaloid Preparations of Uncaria tomentosa on Peripheral Blood Mononuclear Cells . Planta Med. 2004; 70(3):205-10.
23. Keplinger K, Wagner H, Kreutzkamp B. Oxindole alkaloids having properties stimulating the immunologic systems. Washington DC: United States Patent N° 4844901, 1989.
24. Åkesson Ch, Pero RW, Ivars F. C-Med 100, a hot water extract of Uncaria tomentosa, prolongs lymphocyte survival in vivo . Phytomedicine 2003;10(1):23–33.
25. Kim KS, Pham TNN, Jin Ch-J, Umeyama A, Shoji N, Hashimoto T, et al. Uncarinic acid C isolated from Uncaria rhynchoplylla induce differentiation of Th1-promoting dendritic cells through TLR4 signaling . Biomarker Insights. 2011;6:27-38. doi: 10.4137/BMI.S6441.
26. Lozada-Requena, I, Núñez C, Álvarez Y, Aguilar JL. Efecto de un extracto hidroalacohólico de Uncaria tomentosa (uña de gato) sobre la población de células dendríticas y sus moléculas HLA-DR y CD86 ante el estímulo con lipopolisacáridos . Rev Peru Med Exp Salud Publica. 2009; 26(2):168-74.
27. Hunter CA. New IL-12 family members: IL-23 and IL-27, cytokines with divergent functions . Nat Rev Immunol. 2005 Jul;5(7):521-31.
28. Xu M, Mizoguchi I, Morishima N, Chiba Y, Mizoguchi J, Yoshimoto T. Regulation of antitumor immune response by the IL-12 family cytokines, IL-12, IL-23, and IL-27 . Clin Dev Immunol. 2010;2010. pii: 832454. doi: 10.1155/2010/832454.
29. Lemaire I, Assinewe V, Cano P, Awang DVC, Arnason JT. Stimulation of interleukin-1 and -6 production in alveolar macrophages by the neotropical liana, Uncaria tomentosa (Uña de Gato) . J Ethnopharmacol 1999; 64(2):109–115
30. Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy . Nat Rev Immunol. 2010 Apr;10(4):248-56. doi: 10.1038/nri2742.
31. Benevides L, Cardoso CR, Tiezzi DG, Marana HRC, Andrade JM, Silva JS. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor . Eur J Immunol. 2013; 43(6):1518-1528. doi: 10.1002/eji.201242951.
Correspondence: Ivan
Lozada-Requena.