Original Article
Development of a service on line advice and information technology management for health
Victor Berrospi Polo 1,a, Juan Rodriguez Abad 2,a, Juan Bobadilla Aguilar 3,c, Carlos Di Liberto Moreno 2,d, Cecilia Díaz Arroyo 3,e, Carlos Rafael Quipan 3,f
1 Unite Nations Office for Project Services(UNOPS). 2Health Care Consulting S.A.C. Lima, Peru. 3 Individual consultant. Lima, Peru
a Physician, specialist in proyects, bphysician, MBA; c physician, specialist in medical equipment, specialist in management of technology; d electronic engineer, specialist in medical equipment; e obstetrical practitioner, specialist in telemedicine; f systems engineer
ABSTRACT
Objective. To validate an advisory service and online information technology management for health and helps to make assessment and acquisition processes an informed medical equipment according to the market and the needs of the health institutions. Materials and methods. Internet via a technological solution supported on a data base containing systematic and updated information on technical specifications of 25 compared medical equipment, the same reference prices, list of suppliers, agents and / or producers and technical standards are developed. The ”virtual” technical assistance was made with the support of a team of specialists in Health Technology Management, the decision makers in the planning, evaluation and procurement of biomedical equipment. The validation of the service was conducted by involving specialists in the field of Health Technology Management, from different disciplines and institutions who worked in health, public and private. They used the service for a period of time to verify its feasibility of use as well as its usefulness for their planning, evaluation and procurement of biomedical equipment. To these experts we applied a survey before and after them about the software developed in this project. Results. We found that it is common to use the internet to search for information on medical equipment. Also, an increase on the view that the application will help in procurement of biomedical equipment (40% to 78%) was observed, it will improve the information system (40% to 89%) and communication among physicians, nurses, planners, engineers and other professionals involved in this process (20% to 78%). Conclusions. There is a need for a technological tool available with such features contribute to technology management in Peru.
Key words: Development of technologies; Health technology; Information technologies and communication projects (source: MeSH NLM)
INTRODUCTION
It is estimated that more than 4.6 billion soles (approximately 1.53 billion dollars) have been invested in health equipment and infrastructure in Peruvian healthcare institutions during the past 8 years (1,2). According to the PAHO/WHO, new technology is introduced into the market each year, making it difficult to evaluate and identify its real benefits (3,4). On the other hand, technological trends in the healthcare sector are aimed at development of new medical technologies, the growing use of computers, information explosion, increasing automation, the need for retraining, major scientific advances, and the need for new specialists (5).
The age when a biomedical technology was purely commercialized has passed (5). Now its acquisition in hospital environments is a complex process that includes involvement of a multidisciplinary team, market research, and review of the technical specifications of equipment to be purchased (2,4,6). Decisions in this field are generally made without the appropriate technical information (3,4,7) due to the lack of personnel with knowledge of technology management and due to the lack of a systematized “technological memory” (8), particularly in government healthcare; these phenomena can be seen as major shortcomings. This is the reason why—at various healthcare institutions in this country—there is currently limited availability of information that is up-todate, organized, and comprehensive for evaluation and acquisition of medical equipment. This situation means that the same biomedical equipment—that is listed by the same supplier and in the same time period—can be purchased at one price in Lima and up to double that in the provinces (9).
It is estimated that the lack of economic resources in Peru (due to inadequate management) can cause an increase in the price of the equipment (up to twofold), thus reducing the useful life to half of that indicated by the manufacturer (9). Even though there are institutions that commercialize organized and comprehensive information in the international market (10,11), there are no public or private entities that provide this type of information at the local level, except for those that draw up and formulate technical specifications during project implementation. These are often out-of-date copies of databases or verbatim copies of catalogs from a specific brand and determine a reference value on the basis of biased information or historical data.
Therefore, the aim of this study was to develop an informative and advisory online service for healthcare technology management that facilitates processes of evaluation and acquisition of medical equipment in an informed manner according to the market and the needs of healthcare institutions.
MATERIALS AND METHODS
STUDY DESIGN
This was a cross-sectional study. Because the study is focused on analyzing the level or status of one or several variables at a time, a survey was carried out before and after implementation of the proposed software application.
STUDY POPULATION
The analytical unit consisted of a professional specialist with experience in technology management. Therefore, the focus was on identifying professionals with experience in management of medical equipment technology in various companies and disciplines. We included 20 specialized professionals with more than 10 years of experience in technology management. The vast majority was engineers (electronic, electrical, electromechanical, or industrial), and the smallest proportion was economists and doctors with broad experience in acquisition of biomedical equipment.
DEVELOPMENT OF THE TECHNOLOGICAL SOLUTION
Twenty-five pieces of biomedical equipment that were the most frequently purchased by healthcare institutions were selected; the following technical information on this equipment was entered into the database of the technological tools: technical specifications of the equipment, reference prices, suppliers, representatives, and/or manufacturers. A description of technical standards was also included.
The features that each piece of biomedical equipment must have were analyzed. These included: clinical and functional application, target population, technology, and ranges of characteristics or parameters that each of the components must have. The following criteria were used for gathering information: a) name, b) synonyms, c) specialty, d) definition, e) operating principle, f) proper operating conditions, and g) technical specifications of the equipment.
The following sources were reviewed to gather information from the technical specifications: Emergency Care Research Institute (ECRI) (13), Support Program for the Health Sector Reform (PARSALUD II, Spanish acronym) (14), acquisition of medical equipment for ESSALUD (Seguro Social de Salud del Perú) care centers through the United Nations Office for Project Services (15), and processes recently awarded at the national level. Technical specifications for 89 pieces of medical equipment were drawn up by brand and model after obtaining results of the review and analysis.
Reference prices were obtained from the Electronic System of Government Procurement (SEACE, Spanish acronym) website (16). The supplier information was taken from the list of awards from several bidding processes carried out by MINSA (Ministry of Health of Perú), ESSALUD, and regions, among others and from the website of each supplier. Fifteen technical standards related to the equipment and other data of interest on the technical equipment for management of healthcare institutions, taken from the MINSA website and other sources (17), were also obtained.
From January 22 to August 30, 2013, the obtained information was organized, updated, and integrated and then entered into the database of technological solutions. A process for expert online advice was also developed for any user consultation regarding management of medical equipment (technology). Finally, testing was carried out regarding the performance, response time, and security of the resulting software. The software was intended to be user-friendly and easy to navigate. Once the implementation and testing processes were completed, the software was installed on the production servers and was made available to end users. A web storage (hosting) service was contracted for this purpose.
FEATURES OF THE TECHNOLOGICAL SOLUTION
The application resides on a web server and manages and fulfills client requests, sending them to the interpreted-language module and a database that stores the information on items entered into the application. It uses the InnoDB technology to build tables and an interpreted-language module that fulfills the requests from the web server, interpreting them and constructing the response that will be sent to the client.
VALIDATION OF THE TECHNOLOGICAL SOLUTION
The service was validated via participation of specialized professionals from different disciplines who worked in public hospitals and other government entities (Ministry of Health), the private sector (private clinics), and international organizations. These professionals participate in the technological management of biomedical equipment at these locations, including planning, acquisition, and/or maintenance. These professionals used the technological solution for some period to test its user-friendliness as well as its usefulness for planning, evaluation, and acquisition of biomedical equipment.
THE VALIDATION PROCEDURE
The opinions of the aforementioned professionals were gathered before and after the use of the technological tool, and information was provided to them on technical specifications of the medical equipment, the reference prices, standards they are governed by, manufacturers or local suppliers selling them, and other data.
In the entrance survey, they were asked to provide a response on the subjects of planning and acquisition of medical equipment as well as the features that software for technology management should have. They later received our software application via the Internet, along with a password, and were advised to familiarize themselves with it and to get to know its features. Finally, they were asked to respond to an exit survey that was similar to the entrance survey.
DESIGN OF THE SURVEY
A survey containing 23 questions was devised. It contained the following sections: planning, acquisition, the use of the software application for biomedical equipment, the use of the “online query,” ease of use of the application, and perceived usefulness of the application. Previously validated instruments were reviewed for this purpose. One of these was the matrix for evaluating the technological application cycle in comparison with structure levels. This tool was implemented in the Ministry of Health National Eye Institute by the Bioengineering
Department of the Pontifical Catholic University of Peru (PUCP, Spanish acronym) (18).
DATA PROCESSING AND ANALYSIS
The collected data were entered into tables for descriptive analysis of the results. The answers of the participants before and after the use of the technological solution were compared by means of frequencies and percentages.
RESULTS
The results revealed changes in opinion of the professionals surveyed after getting to know and using our technological solution. These opinions were gathered via a survey before and after the use of the technological solution.
Regarding the source of specialized technical information on medical equipment that was used during compilation of the technical specifications, we observed an increase from 61.9% to 66.7% in the use of technical manuals, brochures, and datasheets for the medical equipment, and from 38.1% to 44.4% in the use of the Internet. In contrast, the use of specialized journals decreased from 38.0% to 11.1%, and the use of MINSA technical specifications decreased from 19.0% to 11.1%. None of the professionals used any application.
As for updating of a new technology, there was a 17.5% decrease (from 61.9% to 44%) in the number of information requests to the suppliers and a 7.9% decrease (from 19% to 11.1%) in reviewing of technical manuals. In contrast, a 12.7% increase (from 42.9% to 55.6%) was observed in the use of the Internet, and a slight increase (by 1.6%, from 9.5% to 11.1%) in the use of specialized journals and MINSA information. Similarly, there was an unexpected increase in the use of applications for technology management.
To determine the reference prices of the medical equipment, after getting to know and using our technological solution, the professionals showed an increase by 17.5% for consultations with suppliers, 12.75% for the use of outdated prices, and 11% for the use of applications. We found that the use of the Internet for these purposes did not change. It was also a decrease in the use of specialized journals and an increase in the use of computer applications for technology management. Internet access is growing, and there are now several applications of this kind, some with free access and others requiring payment for membership.
We found that for 53% of the professionals, it took 2 to 4 days to get a reference price, for 15% from 5 to 7 days, and for 25% of the professionals more than 2 weeks. None of them finished the task in one day. The same trend was observed in the exit survey.
There was a 33.3% decrease in the use of databases that had not been updated and a 7.9% decrease in the use of incomplete supplier databases when trying to find information on the list of medical equipment suppliers that makes it possible to compare merchandize by brand, model, and other features. A similar pattern was observed for the use of information scattered among several files and the use of computer applications. Nonetheless, the use of the updated supplier database increased (from 19% to 33%).
There was an increase in consultation of technical manuals and biomedical equipment brochures (9.5%) and an increase in the use of applications (44.4%) regarding sources of up-to-date information on technical bids and equipment prices for specialists that participated in an acquisition committee. These parameters decreased along with confidence of the bidder for the use of the Internet or specialized journals. There were no changes in consultations with a specialist or MINSA.
Regarding the availability of technological solutions for biomedical equipment, we found that there was an increasing trend for their use when searching for technical specifications of biomedical equipment, the reference price, and suppliers of this equipment.
In terms of the features that a technology management application (software) must have, we found that before and after getting to know the application, the participants opined that the software should be easy to navigate and user-friendly and it should be easy to view the data on technical specifications, reference prices, information on suppliers, manufacturers, or local representatives that contains technology management standards and that it should be easy to teach others how to use this software.
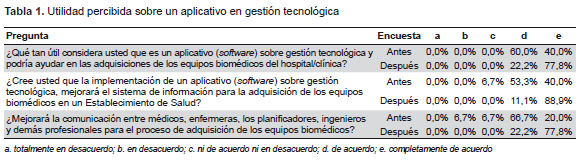
The survey participants strongly agreed that the use of a technological solution like the one tested here can facilitate the process of acquisition of biomedical, hospital, and clinical equipment. The percentage of this opinion increased from 40% to 79% after getting to know our technological solution. Likewise, the professionals said that the use of this application can improve the information system for acquisition of biomedical equipment. The percentage of this opinion increased from 40% to 89% after they familiarized themselves with our technological solution.
Finally, even before getting to know the technological solution, 20% of the survey participants strongly agreed that the use of a technology management application would improve communication among doctors, nurses, planners, engineers, and other professionals in the process of acquisition of biomedical equipment. The percentage of this opinion increased to 78% (Table 1).
DISCUSSION
Biomedical technology is currently one of the key elements of healthcare systems and has important implications for the cost and quality of services (19). It is known that the global expenditures on medical devices increased from $145 billion in 1998 to $220 billion in 2006, i.e., an annual increase by more than 10% (19,20).
Therefore, at the international level, there are organizations that specialize in the evaluation of medical technology that support such evaluation (21). In addition, regarding the process of evaluation of biomedical equipment undertaken in other countries (USA, Sweden, Denmark, Mexico, and Colombia), our efforts to follow standardized processes should allow for better healthcare decisions via multidisciplinary work and the implementation of methods whereby technical and clinical assessments are integrated (4,19,20,22).
For the technological evaluation of medical equipment, defined as a process of reviewing the technical specifications for biomedical equipment, such as the operating, security, and electrical requirements and compatibility (6), there are several sources of information. One of these, at the international level, is various entities (e.g., ECRI, Espicom) (10) that have information on the technical features of biomedical equipment and the healthcare market. These organizations provide the medical community with technical assistance and information on healthcare services. One of the limitations of accessing these sources is that the information on a price is not always available. There is another entity called CENETEC (11) where, although its access is free of charge, the information provided is not always up-to-date. Other sources are those provided by the manufacturers in their brochures, datasheets, and websites. The latter require some searching.
In developing countries, the evaluation of medical technology must be adapted and transformed into an activity aimed at solving problems (not the problems with the technology itself) (19). On the other hand, modern management requires quick, efficient, and effective processes that guarantee quality of the service and/or product in accordance with the established aims and plans, fundamentally based on technological management (20,23,24). The logistics office is not synonymous with the purchasing office. Nevertheless, logically, the latter process is known better. It is not only one process: it consists of several processes that are closely linked to each other, such as selection, programming, acquisition, and storage (25,26).
Acquisition is the purchase itself, for which the logistics team is solely responsible. Nevertheless, a very detailed requirement, including the technical specifications of the medical equipment, had to be formulated in order to implement the purchase. For this purpose, participation of healthcare professionals is vital. Likewise, the bids presented by bidders must be evaluated technically before the award decision is made, an activity in which healthcare professionals should participate (8,25).
The current model of the public sector is mostly a bureaucratic administration system with a formal structure and procedures, control, and documentation that is excessive and unnecessary: an inverted-pyramid hierarchical administration system. As a result, the logistics unit often does not attain the anticipated efficiency level and causes delays in the process of supply of goods and contracting services, thus increasing the operating costs and, in many cases, causing shortages (3,4,12).
It is known that the lack of information on the medical equipment market leads to poor management of acquisition and use of medical equipment with a substantial waste of financial resources. As indicated by the Pan-American Health Organization/World Health Organization, incorrect definition of user requirements, poor purchasing management, inappropriate use, and the lack of training and supplies or responses yield costs on top of the price of biomedical equipment that can add up to 100% of the purchasing price (7,9).
According to our results, there is a tendency among managers to use the Internet and technical manuals as sources of specialized technical information on medical equipment. This is an interesting finding because it will support our proposal here to develop an Internet access tool, even more so if its use continues to increase.
In relation to obtaining technical regulations for medical equipment (6,17), it can be demonstrated that there is still a preference for the MINSA website. An increase in the use of technology management applications and other software was also observed. The same trend was also observed for updating of a new technology. Regarding market research, we found that it is a common practice. This result was corroborated elsewhere (25).
Several sources were used to obtain information on medical equipment suppliers. As mentioned above, these may be brochures, datasheets, or websites of each manufacturer. A tendency to use technology management software was observed here. Likewise, we noticed that more than 38% of the specialists use sources that are not up-to-date and contain incomplete information, 20% use incomplete sources, and a very small percentage uses information that is uptodate and complete.
The Internet and technical manuals for medical equipment are the most widely used sources for verification of technical bids and prices offered by bidders while their bids are presented. Regarding the time it takes to obtain the reference prices, more than 60% of the specialists were found to spend between 2 and 7 days on this process. The use of software may decrease this broad range (25-27).
After the participants reviewed the proposed technical solution, its use—for searching for technical specifications, reference prices of medical equipment, and the information on medical equipment suppliers— increased. This finding may mean that the participants expect to use a technological tool if it is available. This notion was confirmed here: when our software application was made available to the professionals, the interest in its use increased from 80% to 100%.
After the professionals saw the proposed application, we observed an increase in the frequency of use of online technical consultations on medical equipment.
As for the features that a technology management application should have, the ones most valued by the participants, after the presentation, were the ease of understanding the options; availability of information on suppliers, manufacturers, and local representatives of medical equipment; the presence of standards related to healthcare technology management; and the ease of teaching others how to use it. It is worth mentioning that our software application already has the majority of the features indicated by the participants.
Finally, a significant improvement was observed in the opinion of the participants when considering whether the technology management application would be useful when purchasing biomedical equipment (from 40% to 89%) and in communication among doctors, nurses, planners, engineers, and other professionals participating in this process (from 20% to 78%).
It should be noted that one of the advantages of our technological solution is availability of up-to-date information on the technical features of the most recent brands and models of biomedical equipment that constantly changes with advances in technology and an online consultation service on topics related to biomedical equipment. Even though this study covers only a small range of biomedical equipment, this range is expected to gradually increase in order to cover the greatest number of instruments and to enable integration with the rest of hospital technology at a significantly lower cost than international prices. This improvement will allow for savings instead of the costs that are entailed by a poorly planned purchase.
Although this study has limitations in representation due to the limited sample size, these results, according to the validation of our technological solution, allow us to conclude that there is a need for a software application with these features that clarifies features of the medical equipment market, thus allowing for more objective decisions during the process of planning and acquiring medical equipment in Peru. To obtain more conclusive results, it is worthwhile to continue validating similar technological tools with more complete information that is always up-to-date in larger and more representative populations.
Author contributions: JRA, JBA, VBP, and CDLM participated in planning of this study, collection of data, editing of the manuscript, and approval of the final version. CRQ and CDA added various data to the application, and JRA obtained the funding.
Sources of funding: Contract 037: Fund for Innovation, Science and Technology (FINCyT), FIDECOM-PIMEN-2012.
Conflicts of interest: the authors declare that there are no conflicts of interest
REFERENCES
1. Berrospi Polo V. Tendencias de la inversión en equipamiento de hospitales del Ministerio de Salud de Perú: 1995-2006 . En: Federación Peruana de Administradores de Salud. III Congreso Latinoamericano de administradores de salud & I Congreso Peruano de Administración Hospitalaria. Lima: FEPAS; 2007. p. 30.
2. Perú, Ministerio de Salud. Documento técnico: Lineamientos para la elaboración del plan de equipamiento de establecimientos de salud . Lima: MINSA; 2012.
3. Organización Panamericana de Salud. Evaluación de tecnologías en salud: metodología para países en desarrollo . Wasington D.C: OPS; 1990.
4. Colombia, Ministerio de Salud. Manual de Adquisición de Tecnología Médica [Internet]. Santa Fe de Bogotá: Dirección de Desarrollo Científico y Tecnológico; 1997 [citado el 15 de mayo de 2015]. Disponible en: http://www.saludcordoba.gov.co/portal/descargas/tecnologiabiomedica.pdf
5. Nones de Osorio N. Gestión tecnológica en el sector salud. Caso: Centro Médico Paraíso . Telos 2001;3(2):117-27.
6. Carvajal M, Ruiz C. Evaluación técnica y clínica de tecnología biomédica en procesos de adquisición: un enfoque en evaluación de tecnologías en salud . Rev Ing Bioméd. 2008 Jul;2(4):34-45.
7. Flores G. Como disminuir los accidentes en la atención de salud mediante calidad total, uso de computadoras y otras medidas . Rev Latinoam de Derecho Médico y Medicina Legal. 2002 Dic;7(2) - 2003 Jun;8(1):43-54.
8. Perú, Ministerio de Salud. Programa de Fortalecimiento de Servicios de Salud (PFSS). Diagnóstico de los sistemas de logística. Serie: Informes técnicos [Internet]. Lima: MINSA; 1999 [citado el 10 agosto de 2014]. Disponible en: http://www.minsa.gob.pe/publicaciones/pdf/diagnosticolog.pdf
9. Vilcahuamán L, Rivas R. Ingeniería clínica y gestión de tecnologías en salud: avances y propuestas . Lima: CENGETS-PUCP; 2006.
10. Espicom Business Intelligence [internet]. London. Overview of the medical market in Peru [citado el 15 de mayo de 2015]. Disponible en: http://www.espicom.com/peru-medical-device-market.html#sthash.LwGb7h4n.dpf
11. cenetec.salud.gob.mx [internet]. México D.F.: Centro Nacional de Excelencia Tecnológica en Salud [citado el 15 de mayo de 2015]. Disponible en: http://www.cenetec.salud.gob.mx/contenidos/conocenos/conocenos.html
12. García SP, Zuleta DA. Diseño e implementación de una metodología para la evaluación biomédica instalado: unidad de urgencias y unidad de cuidado intensivo pediátrico, hospital universitario del Valle "Evaristo García" . Tesis para optar el grado de Ingeniero Biomédico. Facultad de Ingeniería, Universidad Autónoma de Occidente. Santiago de Cali, Colombia. 2011.
13. Emergency Care Research Institute (ECRI). Select Plus: Healthcare Product comparisons (Internet). 2014. Disponibe en: https://www.ecri.org/Pages/default.aspx . Consultado el 15 de mayo 2015. (IGUAL AL N°12)
14. Perú, Ministerio de Salud. Programa de Apoyo a la Reforma del Sector Salud. Revisión de las especificaciones técnicas de los equipos médicos adquiridos. Lima: MINSA; 2006.
15. Seguro Social de Salud. Revisión de las especificaciones técnicas de equipos médicos adquiridos, en el periodo: 2008-2013. Lima: EsSalud.
16. Organismo Supervisor de las contrataciones del Estado. Ley de contrataciones del Estado. Lima: OSCE; 2014.
17. Perú, Ministerio de Salud. Normas técnicas de Salud [Internet]. Lima: MINSA [citado el 15 de mayo de 2015]. Disponible en: http://www.minsa.gob.pe/serumsBVS/SupportFiles/normas.htm
18. Vilcahuaman L, Tovar J, Callupe R, Almeyda E. Evaluación de la capacidad de gestión tecnológica del Instituto Nacional de Oftalmología, INO-MINSA . Lima: Pontificia Universidad Católica del Perú; 2000.
19. Banta D. An approach to the social control of hospital technologies . Ginebra: OMS; 1995.
20. Organización Mundial de la Salud. Formulación de políticas sobre dispositivos médicos (serie de documentos técnicos de la OMS sobre dispositivos médicos) . Ginebra: OMS; 2012.
21. rganización Mundial de la Salud. Evaluación de tecnologías sanitarias aplicadas a los dispositivos médicos (srie de documentos técnicos de la OMS sobre dispositivos médicos) . Ginebra: OMS; 2012.
22. Secretaria de Salud de México. Procedimiento para evaluación y adquisición de tecnología médica . México D.F: Centro Regional de Alta Especialidad de Chiapas; 2008.
23. Organización Mundial de la Salud, Organización Panamericana de la Salud. Conferencia Internacional de Autoridades Reguladoras de Equipos Médicos. Ginebra: OMS; 1996.
24. Organización Mundial de la Salud. Evaluación de las necesidades de dispositivos médicos (serie de documentos técnicos de la OMS sobre dispositivos médicos) . Ginebra: OMS; 2012.
25. Perú, Ministerio de Salud. Programa de Fortalecimiento de Servicios de Salud. Curso de gestión en las redes de establecimientos y servicios de salud: Gestión Logística . Lima: MINSA; 1998.
26. United Nations Office for Project Services. Manual de Adquisiciones. Rev 2, diciembre 2007. Mexico D.F: UNOPS; 2007.
27. Organización Mundial de la Salud. Guía de recursos para el proceso de adquisición. Serie de documentos técnicos de la OMS sobre dispositivos médicos . Ginebra: OMS; 2012.
Correspondence: Victor Hugo Berrospi Polo
Address: Av. Central 960, Cond. El Prado B5102, Álamos de Monterrico, Surco. Lima, Peru
Phone number: (511) 996443485
E-mail: berrospipolo@gmail.com
Received: 9/25/2014
Approved: 6/17/2015