ORIGINAL ARTICLE
Antimicrobial activity in vitro of camu-camu (Myrciaria dubia) against oral microorganisms: a systematic review
Karina Pardo-Aldave 1,a, María Pareja-Vásquez 1,b, Alfredo Guillén 2,c, Juan Manuel Ureta-Tapia 3,d
1 Facultad de Odontología. Universidad de San Martin de Porres. Lima, Perú.
2 Universidad Nacional Federico Villarreal. Lima, Perú.
3 Instituto Nacional de Salud. Lima, Perú.
a
Dental Surgeon. Master's Degree in Stomatology; b Dental Surgeon. PhD in Education; c Physician; d Physician. Specialization in Field Epidemiology.
Master's Degree in Public Health, Epidemiology.
ABSTRACT
Objectives. To evaluate the antimicrobial activity of Myrciaria dubia on oral microorganisms. Materials and Methods. A systematic review of the literature following PRISMA guidelines was conducted through searches of studies published between 2008 and 2018 in Pubmed, LILACS, SciELO, ProQuest, EBSCO, and Google Scholar. Results. Eleven (11) in vitro studies were gathered; all the studies showed positive antimicrobial activity on gram-positives, mainly by each of the parts of its fruits. However, such activity compared to chlorhexidine in only two studies, and, in another study, it was better than an antibiotic. A high risk of bias was detected in most studies. Phenolic compounds, including polyphenols and acylphloroglucinols, were identified as responsible for its activity. Conclusions. There is evidence of antimicrobial activity in M. dubia. Its study as an antimicrobial against oral microorganisms is still incipient, but there is great potential thanks to the potent phytochemicals it contains. Also, additional quality studies are required: comparing their activity versus oral antiseptics and on microorganisms associated with dental caries and periodontal disease.
Keywords: Review; Phytotherapy; Myrtaceae; Dental caries; Microbiology; Periodontitis (source: MeSH NLM).
INTRODUCTION
Dental plaque (DP) at supra and subgingival level is linked to gingivitis, dental caries (DC) and periodontal disease (PD), respectively (1). Its main pathogens are Streptococcus mutans (S. mutans) and Porphyromonas gingivalis (P. gingivalis) (2,3). To maintain healthy oral tissues, toothbrushes, toothpastes, dental floss, and oral antiseptics (4-6) are required. Chlorhexidine (CHX) gluconate is the first choice (7) because it combines antibiotic/antibacterial effects. However, it is used for a short time because it results in teeth staining and temporary taste disorder (8,9). Today, phytotherapy has a potentially valuable role as an adjunct in DC and DP management.
Myrciaria dubia (H.B.K) (McVaugh) "camu camu", is a fruiting shrub native to the Amazon region that belongs to the Myrciare family. It accumulates phytochemicals which are related to important properties (10). For example, fractions of hydro-methanolic and hydro-acetonic extracts, as well as crude extract from M. dubia seed (11) and skin showed antioxidant activity due to their C-glucosidic ellagitannins (11,12). Also, the pulp showed high levels of ascorbic acid (AA) and a large number of phenolic compounds (13). The aqueous extract of the seed cover of M. dubia was also high in phenolic content (14).
In addition, a methanolic extract of M. dubia seeds showed anti-inflammatory properties by suppressing edema in mouse legs (15) and a 100% M. dubia juice reduced inflammatory markers and IL-6 and IL-8 in smokers with accelerated oxidative stress (16). Betulinic acid (triterpenoid anti-inflammatory) (15) AA, and unknown phytochemicals (16) are responsible for this property, respectively.
Probiotic beverages enriched with phenolic compounds of M. dubia controlled early stages of type 2 diabetes and the risk of associated hypertension (17), and an extract of seed cover showed antihypertensive activity (14). In addition, crude extract (18) and pulp (19) from M. dubia controlled obesity (18,19) in mice (18) and rats (19), respectively. Also, a dry extract from pulp + shell stopped the growth of tumors and did not develop a greater inflammatory response in rats with colorectal cancer (20). Finally, an aqueous extract of M. dubia pulp showed immunostimulant activity in rats (21).

There is research proving its antimicrobial property, mainly in the areas of Food Science, Chemical Engineering and Microbiology. But studies in dentistry are incipient. To date, there are no systematic reviews studying the antimicrobial activity of M. dubia versus oral antiseptics on oral cavity microorganisms. DC and PD are complex chronic diseases induced by pathogenic DP (22). For this reason, agents that inhibit the growth of oral microorganisms should be studied in order to develop new preventive/therapeutic formulations. Proper mechanical control of the accumulation of DP in teeth has been key for preventing such diseases, but it requires great cooperation and motivation from the patient. Therefore, chemical agents act as useful adjuncts to achieve effective management of dental plaque associated with DC and PD (23). Consequently, the objective of this study was to evaluate the in vitro antimicrobial activity of M. dubia against oral microorganisms.
MATERIALS AND METHODS
RESEARCH QUESTION
This systematic review was designed according to PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (24) to answer the question: does Myrciaria dubia (Intervention) exercise true antimicrobial activity (Event/Outcome), compared with positive and/or negative controls (Comparison), on oral microorganisms (Problem) in experimental studies in vitro (Study design)?, which was formulated according to the PICO format.
SELECTION CRITERIA
Types of studies. In vitro experimental studies published in the last ten years (January 1, 2008 to December 31, 2018) because they are the most recent, rigorous and contain the most relevant data. Studies researching the antimicrobial activity of M. dubia versus controls, against oral microorganisms, without restriction of language and geographical scope.
Types of participants. Oral microorganisms involved in the etiology and progression of DC and PD (Streptococcus mutans, Streptococcus sanguinis, Streptococcus sobrinus, Candida albicans, Porphyromonas gingivalis, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Tannarella forsythia, Treponema denticola or Staphylococcus aureus) using cultures of strains or samples of saliva or dental plaque from humans.
Types of intervention. Extracts (alcoholic, hydroalcoholic and aqueous), extract fractions, isolated phytochemicals, juices and/or antiseptics (solutions) from the fruit or tissues/ organs of M. dubia versus positive controls (chlorhexidine, chloramphenicol, commercial oral antiseptics, antibiotics and antifungals) and negative controls (placebo solutions).
Types of event measurements. Primary event: antimicrobial activity determined by antimicrobial susceptibility methods, agar diffusion (disc or well), and broth microdilution method. The results were quantified by measuring the microbial growth inhibition zone (mm) and minimum inhibitory concentration (MIC), respectively. Secondary event: Identification of phytochemicals responsible for the antimicrobial activity of M. dubia.
EXCLUSION CRITERIA
Types of studies. Literature reviews, case reports, projects/ protocols, short communications, personal opinions, letters, posters, conference summaries, and in vivo studies.
Types of intervention. Pharmaceuticals (such as tablets, capsules) or extracts containing combinations of M. dubia with other pharmaceuticals or other medicinal plants.
SEARCH METHODS FOR THE IDENTIFICATION OF STUDIES
Database information: The search for scientific articles was carried out in electronic databases for each of the predefined interventions between July 11 and September 15, 2018. No language limits were applied, and foreign articles were translated.
Databases and search engines used were PubMed; ProQuest; SciELO (Scientific Electronic Library Online); LILACS (Latin American and Caribbean Health Sciences Literature); EBSCOhost, Google Scholar.
Search strategies. A search model was developed for PubMed, using controlled MeSH (Medical Subject Headings) and free terms. For the other databases, this model was adapted, and free terms based on the controlled terms from MeSH or DeCS (Health Sciences Descriptors) and/or a combination of controlled vocabulary with free terms were used, taking into account differences in controlled vocabulary and syntax rules (Appendix 1).
DATA EXTRACTION AND ANALYSIS
Data extraction. In each article retrieved through the search strategies, two authors (KPA and MPV) applied the selection/exclusion criteria to the abstract, title or both, and reviewed them simultaneously and independently; only studies raising doubt were fully read. All potentially relevant studies were searched in full text. Those which did not meet the selection criteria were registered as articles excluded from the review and the reason for their exclusion was noted. Any discrepancies were resolved through consensus with a third and fourth reviewers (GA and JMUT).
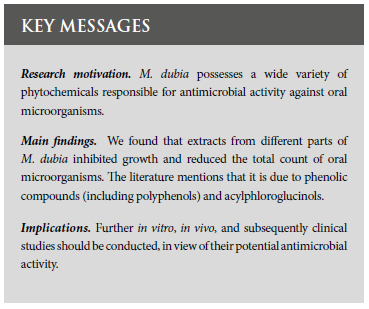
The primary and secondary events were the antimicrobial activity of M. dubia and the identification of phytochemicals responsible for this activity, respectively. Additionally, information was extracted on aspects such as the name of the main author, year of publication, country of origin of the study, pharmacological formulation, part/tissue/organ and origin of M. dubia, control group, oral microorganisms studied and method to evaluate antimicrobial activity. Figure 1 shows the selection process of the studies according to the PRISMA statement. Information on the antimicrobial activity of M. dubia and controls was also extracted.
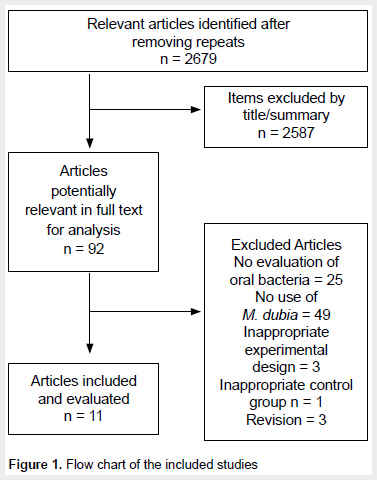
Risk of bias assessment of the studies included. Two researchers (KPA and AG) independently assessed the risk of bias of the selected studies, following the criteria proposed by the Joanne Briggs Institute (Joanna Briggs Institute, 2014) (25). A third reviewer was involved in case of discrepancy (JMUT). The scale has ten questions, but two were not considered because they did not comply with bias analysis for in vitro studies. Therefore, the following questions were evaluated:
Was allocation to treatment group truly random? Was allocation to treatment groups hidden from the allocator? Were outcome assessors blinded to treatment allocation? Were control and treatment groups comparable at baseline? Were groups treated identically by the interventions mentioned? Was the event measured in the same way for all groups? Was the event measured reliably? Was the statistical analysis used appropriate?
Each question was answered with a "yes" when sufficient information was available, being equivalent to a low risk of bias. In case of non-existent information, the answer "no" was given, which is equivalent to a high risk of bias. The response "unclear" was given when the risk of bias could not be classified as high or low (Figure 2).
Looking for heterogeneity. It was determined according to the intervention (characteristics of the M. dubia and types of controls) and the event (methodology) studied in each of the selected articles. Thus, a meta-analysis could not be performed because the articles showed high heterogeneity, making it impossible to make similar comparisons to assess the main event.
RESULTS
Four thousand nine hundred eight (4908) titles/abstracts were retrieved, of which 2229 were excluded because they were duplicated. Based on the selection criteria, 92 full titles were evaluated and 11 were selected (26-36). The reasons for exclusion are mentioned in Figure 1.
The main characteristics for which the studies were selected, and their most relevant results are presented in Table 1.
Different parts of M. dubia were used (in correlative order,(26-30), pulp (31-33), seed (26,29,33), leaves (34,35), bark (34), a skin + seed combination) (36) and four phytochemicals (28,29) were isolated. All studies were in vitro (26-36) and one of them included an in vivo (35). The two methods applied to evaluate antimicrobial activity against oral microorganisms (primary event) were broth microdilution (27-34,36) and agar diffusion (26,27,30,34,35) or well (31-33,36)), whose results quantified the minimum inhibitory concentration (MIC) (27-34,36) and the microbial growth inhibition zone (26,27,30-36), respectively. The least used method was the colony forming unit (CFU) (30). Phenolic and lipophilic compounds, polyphenols and acylphloroglucinols were identified as the phytochemicals responsible for the antimicrobial activity of M. dubia (secondary event).
M. dubia came mainly from Peru (26-30,33-35) and Brazil (31,32,36), whereas its most used phytopharmacological formulations were alcoholic (ethanol (27,30), hexane (28,29), and methanol (33)), hydroalcoholic (26,31,34,35) and aqueous (32,36) extracts, whose effects were studied against S. aureus (26-29,31,32,34,36), S. mutans (28-30,33,35), C. albicans (27-29), P. gingivalis (35) and S. sanguinis (33). It also shows that the application of a M. dubia mouthwash on volunteer subjects reduced the microbial flora count up to ten minutes. In addition, it is important to mention that the studies carried out showed positive results with respect to the safety of M. dubia, since no visible adverse reactions were mentioned in the use of a leaf mouthwash in humans (35), and methanolic extracts from seeds and pulp were not cytotoxic in high concentrations (33).
Nine studies used, predominantly, an antibiotic as a positive control (ampicillin (31,32,34,36), kanamycin (26,28,29), vancomycin (27) and penicillin (30)); one of them included fluconazole (27) and another one, an alcohol (only indicated that it was 96°) (35), finding that M. dubia.'s activity was mostly similar (29,30) or superior (28,29,31,33,36) to such antibiotics. Two studies used chlorhexidine (CHX) (in solution) as a positive control (33,35), and one study used a negative control. Finally, the statistical analysis of such study found no significant difference between the activity of four fractions of an extract from M. dubia skin versus penicillin (30).
Study bias was assessed using the criteria described by the Joana Briggs Institute (25), used in systematic reviews of in vitro studies (37-39). Figure 2 shows the risk of bias found in the studies included in this review. Analysis showed that all studies had an 'unclear' risk of bias for randomization, concealment of the randomization list, and blinding of assessors. Regarding an appropriate statistical analysis, it was only conducted in one of the eleven selected studies ("yes") (30). Four studies did not require statistical analysis because they generated results through point estimation (26,28,29,35). In addition, in five studies, statistical analysis was 'unclear' (27,31-33,36) and one did not use appropriate statistical analysis (34) ('no'). The other guidelines, which include baseline comparison between experimental groups, identical treatments, and event measurements were conducted reliably and comparably, showing a "yes" for low risk of bias in all studies.
In one of the studies, it was observed that despite the loss of phytochemicals in the pulp of M. dubia after it was subjected to dehydration processes, the pulp dust still maintained high levels of phenolics, ascorbic acid, proanthocyanidins, and antimicrobial activity (31). An article also concluded that seed and skin extracts inhibited S. aureus, owing to the action of lipophilic constituents (26). Two other publications demonstrated the antimicrobial activity of phenolic compounds of M. dubia; in the first, they identified these compounds in freeze-dried pulp and found that they had a high correlation coefficient with the inhibition of S. aureus (r2 = 0.906 – 0.971) (32). And in the second, Powder from M. dubia seed + shell presented phenolic compounds and polyphenols obtained by freeze-drying and hot air, respectively, indicating that they could play a role in inhibiting S. aureus (36). Finally, two studies isolated four acylphloroglucinols and antimicrobial phytochemicals in the skin (28,29) and seeds (29) of M. dubia, respectively, which showed strong antimicrobial activities against five gram positive bacteria, including S. mutans and S. aureus (28,29).
DISCUSSION
This systematic review showed, for the most part, that M. dubia has antimicrobial activity against microorganisms, gram-positives (G+) (26-36), only one gram-negative (G-) (35), and one fungus (27) from the oral cavity. Also, against G- microorganisms that do not belong to the oral cavity (28,29,40), which suggests that M. dubia could inhibit G- pathogens relevant to the development of PD. Therefore, it is necessary to carry out more in vitro studies on other G+ and G- bacteria linked to DC and PD, respectively, applying other methodologies/techniques and with chlorhexidine as the control group. Then, studies should be conducted in an in vivo environment, such as the mouth. In particular, its substantivity could be studied, which is the property that allows its therapeutic action to persist for as long as possible in the oral cavity. Therefore, further in vitro, in vivo, and later clinical studies should be conducted in view of its potential antimicrobial activity.
M. dubia has traditionally been used by indigenous communities in Loreto (Peru) to treat gingivitis (41) and because it keeps gums healthy (42). Nowadays, it is considered a super fruit because it has several phytochemicals and improves people's quality of life. In recent years, extracts of the whole fruit and its parts have been studied, demonstrating their effects against different pathologies, including their antimicrobial action against oral pathogens (43). Studies on oral microorganisms suggest the antimicrobial activity of M. dubia (26-36).
Strict anaerobic bacteria were studied, as they are considered the main etiological agents of PD (44), but also G+ bacteria—for being causal agents of the CD (45)—and other microbial components of PD. In particular, it was decided to evaluate the antimicrobial activity because it is specific to microorganisms of the oral cavity. For example, the polymicrobial community’s representative of DC and periodontitis show a sophisticated structural and functional integration, conferring an almost organic state to these microorganisms (46). Other properties, such as anti-inflammatory activity, were not studied because it is not specific to the oral cavity and can be evaluated by other experimental models frequently applied to the whole organism.
In relation to the bias analysis of the studies included, there is a perception of bias in most of them because the guidelines for randomization, concealment of the randomization list, and blinding of assessors were not met. However, these guidelines are not normally followed in in vitro microbiological research. With respect to the other guidelines, they were applied, except for statistical analysis, which was not complied with in all the studies as they did not compare experimental groups versus positive (26-29,31-36). In others, it was not required because the method used to measure the main event generated results through point estimation (presented a single value) (26,28,29,35). However, the results would show us the potential to generate evidence.
The mechanism of action of M. dubia’s phytochemicals identified in this review and usually studied as new antimicrobial constituents (28,29,47), is still unknown. However, it has been shown that G+, like S. aureus, showed microbial sensitivity to phenolic compounds from fruits, which act through various mechanisms, including cell membrane destabilization and inhibition of key enzymes (47).
A number of phenolic compounds were the best choice as a supplement during antibiotic therapy because they accelerated antimicrobial activity and inhibited the overproduction of reactive oxygen species by antimicrobial agents (48). In addition, animal studies indicated that many of them improved or prevented the development of PD in terms of inflammation and periodontal destruction (49). Lipophilic compounds would be relevant for the potential of two extracts to prevent dental erosion (50). The term polyphenols are often used as a synonym for phenolic compounds, but it only refers to molecules bearing at least two phenolic rings (51). In vitro (52) cell biology studies in animals and humans have shown that selected dietary polyphenols have important antimicrobial, antioxidant, and anti-inflammatory properties that improve the clinical markers of periodontitis (53). Finally, acylphloroglucinols derived from various medicinal plants exhibited antimicrobial and antifungal activities (54) and possess important structural characteristics that confer antimicrobial activities against resistant strains of S. aureus (55).
Certain limitations were encountered in the present review due to heterogeneity in the intervention and outcomes. In the first, it refers to differences in the M. dubia with respect to the place of origin, type of extract, parts of the fruit used, and different positive controls; in the second, to differences in the methodology for quantifying antimicrobial activity and in its units of measure. In addition, as the results were based on experimental in vitro studies, they do not lead to direct clinical application, but do constitute a necessary prior step. Despite of the limitations in intervention and results, the antimicrobial activity of M. dubia was generally similar (29,30) and/or superior (28,29,31,32,36) to antibiotics, suggesting its antimicrobial potential in the oral cavity, although in the only two studies where it was compared with CHX (33,35) it showed no superiority. This indicates the need for further study in the dental field and thus be able to measure its antimicrobial activity versus conventional oral antiseptics. It is worth mentioning that there are reviews of M. dubia (56,23,57), but this is the first systematic review that evaluates its antimicrobial activity against microorganisms in the oral cavity.
It can be concluded that M. dubia has antimicrobial potential to control dental caries and periodontal disease in the oral cavity, in addition to reducing the total count of microorganisms. In order to corroborate its antimicrobial effect, it should be studied in other formulations such as antiseptics (solutions), toothpastes, chewing gum, etc. The poor methodological design of the studies included in this systematic review does not allow its direct use as an antimicrobial in the oral cavity. We recommend conducting more studies with better design and low risk of bias.
Authors' Contributions: KPA participated in the conception and design of the article, data collection, writing and critical review of the article, analysis and interpretation of the results, and approval of the final version. MPV participated in data collection, critical review of the article, and approval of the final version. AG participated in data collection, article writing, critical review of the article, and approval of the final version. JMUT participated in the data collection, critical review of the article, and approval of the final version.
Funding: Self-funded
Conflicts of Interest: The authors declare that they have no conflicts of interest in relation to the contents of this document.
Supplementary Material: Available in the electronic version of the RPMESP.
REFERENCES
1. Doherty R. Biofilms: What does subgingival plaque look like?. Br Dent J. 2016 Jul;221(1):16. doi: https://doi.org/10.1038/sj.bdj.2016.487.
2. Garcia SS, Blackledge MS, Michalek S, Su L, Ptacek T, Eipers P. Targeting of Streptococcus mutans biofilms by a novel small molecule prevents dental caries and preserves the oral microbiome. J Dent Res. 2017;96(7):807-14. doi: https://doi.org/10.1177/0022034517698096.
3. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30-44. doi: https://doi.org/10.1038/nri3785.
4. Wilder RS, Bray KS. Improving periodontal outcomes: merging clinical and behavioral science. Periodontol 2000. 2016;71:65-81. doi: https://doi.org/10.1111/prd.12125.
5. Mouchrek Junior JC, Nunes LH, Arruda CS, Rizzi Cde C, Mouchrek AQ, Tavarez RR, et al. Effectiveness of oral antiseptics on tooth biofilm: a study in vivo. J Contemp Dent Pract. 2015 Aug;16(8):674-8. doi: https://doi.org/10.5005/jp-journals-10024-1739.
6. Wessel SW, van der Mei HC, Maitra A, Dodds MW, Busscher HJ. Potential benefits of chewing gum for the delivery of oral therapeutics and its possible role in oral healthcare. Expert Opin Drug Deliv. 2016;3:1-11. doi: https://doi.org/10.1080/17425247.2016.1193154.
7. Supranoto SC, Slot DE, Addy M, Van der Weijden GA. The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration. Int J Dent Hyg. 2015;13(2):83-92. doi: https://doi.org/10.1111/idh.12078.
8. Tartaglia GM, Kumar S, Fornari CD, Corti E, Connelly ST. Mouthwashes in the 21st century: a narrative review about active molecules and effectiveness on the periodontal outcomes. Expert Opin Drug Deliv. 2017;14(8):973-82. doi: https://doi.org/10.1080/17425247.2017.1260118.
9. Kulik EM, Waltimo T, Weiger R, Schweizer I, Lenkeit K, Filipuzzi-Jenny E, et al. Development of resistance of mutans streptococci and Porphyromonas gingivalis to chlorhexidine digluconate and amine fluoride/stannous fluoride-containing mouthrinses, in vitro. Clin Oral Investig. 2015;19(6):1547-53. doi: https://doi.org/10.1007/s00784-014-1379-y.
10. Castro JC, Maddox JD, Cobos M, Imán SA. Myrciaria dubia "camu camu" fruit: health-promoting phytochemicals and functional genomic characteristics. Breeding and Health Benefits of Fruit and Nut Crops; London: IntechOpen; 2018. doi: https://doi.org/10.5772/intechopen.73213.
11. Kaneshima T, Myoda T, Toeda K, Fujimori T, Nishizawa M. Antioxidative constituents in camu-camu fruit juice residue. Food Sci Technol Res. 2013;19(2):223-8. doi: https://doi.org/10.3136/fstr.19.223.
12. Kaneshima T, Myoda T, Nakata M, Fujimori T, Toeda K, Nishizawa M. Antioxidant activity of C-Glycosidic ellagitannins from the seeds and peel of camu-camu (Myrciaria dubia). LWT-Food Sci Technol. 2016;69:76-81. doi: https://doi.org/10.1016/j.lwt.2016.01.024.
13. Cunha-Santos ECE, Viganó J, Andrade-Neves D, Martínez J, Teixeira-Godoy H. Vitamin C in camu-camu [Myrciaria dubia (H.B.K.) McVaugh]: evaluation of extraction and analytical methods. Food Res Int. 2019;115:160-6. doi: https://doi.org/10.1016/j.foodres.2018.08.031.
14. Fidelis M, Santos JS, Escher GB, Vieira do Carmo M, Azevedo L, Cristina da Silva M, et al.. In vitro antioxidant and antihypertensive compounds from camu-camu (Myrciaria dubia McVaugh, Myrtaceae) seed coat: A multivariate structure-activity study. Food Chem Toxicol. 2018;120:479-90. doi: https://doi.org/10.1016/j.fct.2018.07.043.
15. Yazawa K, Suga K, Honma A, Shirosaki M, Koyama T. Anti-inflammatory effects of seeds of the tropical fruit camu-camu (Myrciaria dubia). J Nutr Sci Vitaminol (Tokyo). 2011;57(1):104-7. doi: https://doi.org/10.3177/jnsv.57.104.
16. Inoue T, Komoda H, Uchida T, Node K. Tropical fruit camu-camu (Myrciaria dubia) has anti-oxidative and anti-inflammatory properties. J Cardiol. 2008;52(2):127-32. doi: https://doi.org/10.1016/j.jjcc.2008.06.004.
17. Fujita A, Sarkar D, Genovese MI, Shetty K. Improving anti-hyperglycemic and anti-hypertensive properties of camu-camu (Myriciaria dubia Mc. Vaugh) using lactic acid bacterial fermentation. Process Biochem. 2017;59(B):133-40. doi: https://doi.org/10.1016/j.procbio.2017.05.017.
18. Anhê FF, Nachbar RT, Varin TV, trottier J, Dudonné S, le Barz M, et al. Treatment with camu-camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energ y expenditure in diet-induced obese mice. Gut. 2019;68(3):453–64. doi: http://dx.doi.org/10.1136/gutjnl-2017-315565.
19. Nascimento OV, Boleti APA, Schwertz M, Lima ES. Dietary supplementation with camu-camu and continuous exercises in the treatment of ity. v Nutr. 2018;31(1):25-33. doi: https://doi.org/10.1590/1678-98652018000100003.
20. Asmat-Aguirre SN, Benites-Carranza CY. Efecto del fruto de Myrciaria dubia (H.B.K) Mc Vaugh sobre cáncer colorrectal inducido en Rattus Norvegicus Var. Albinus [Tesis]. Trujillo, Perú: Universidad Nacional de Trujillo, Facultad de Farmacia y Bioquímica; 2017. Disponible en: http://dsapce.unitru.edu.pe/handle/UNITRU/7437.
21. Macedo-Ríos R , Mendoza-Acuña JB. Actividad inmunoestimulante del fruto de Myrciaria dubia H.B.K Mc Vaugh "Camu camu", en ratas albinas Holtzmann, Iquitos 2015 [Tesis]. Iquitos, Perú: Universidad Nacional de la Amazonía Peruana, Facultad de Farmacia y Bioquímica; 2015. Disponible en: http://repositorio.unapiquitos.edu.pe/handle/UNAP/3868.
22. Chapple IL, Bouchard P, Cagetti MG, Campus G, Carra MC, Cocco F. Interaction of lifestyle, behavior or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl. 18):S39–S51. doi: https://doi.org/10.1111/jcpe.12685.
23. Jafer M, Patil S, Hosmani J, Bhandi SH, Chalisserry EP, Anil S. Chemical plaque control strategies in the prevention of biofilm-associated oral diseases. J Contemp Dent Pract. 2016;17(4):337-43. doi: https://doi.org/10.5005/jp-journals-10024-1851.
24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and Elaboration. J Clin Epidemiol [Internet]. 2009 Oct [cited 2019 Set 01];62(10):e1-e34. Available from: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000100. doi: https://doi.org/10.1371/journal.pmed.1000100.
25. The Joanna Briggs Institute. Joanna Briggs Institute reviewer’s manual 2014 [Internet]. South Australia: The Joanna Briggs Institute; 2014 [cited 2019 Ago 18]. Available from: https://nursing.lsuhsc.edu/JBI/docs/ReviewersManuals/Economic.pdf.
26. Myoda T, Fujimura S, Park B, Nagashima T, Nakagawa J, Nishizawa M. Antioxidative and antimicrobial potential of residues of camu-camu juice production. J Food Agri Environ. 2010;8(2):304-7.
27. Castillo-Carranza CN, Mejía-Delgado EM. Efecto inhibitorio in vitro de Myrciaria dubia "camu-camu" sobre Staphylococcus aureus y Candida albicans [Tesis Bachiller en medicina]. Trujillo: Universidad Nacional de Trujillo; 2013. Disponible en: http://dspace.unitru.edu.pe/handle/UNITRU/232.
28. Kaneshima T, Myoda T, Nakata M, Fujimori T, Toeda K, Nishizawa M. Rhodomyrtone, an antimicrobial acylphloroglucinol, in the peel of Myrciaria dubia (Camu-camu). Food Preserv Sci. 2015;41(2):71-6.
29. Kaneshima T, Myoda T, Toeda K, Fujimori T, Nishizawa M. Antimicrobial constituents of peel and seeds of camu-camu (Myrciaria dubia). Biosci Biotechnol Biochem. 2017;81(8):1461-5. doi: https://doi.org/10.1080/09168451.2017.1320517.
30. Saldarriaga-Mostacero EG. Efecto antibacteriano in vitro del extracto etanólico de Myrciaria dubia (camu camu) sobre Streptococcus mutans (ATCC 25175) [Tesis]. Trujillo, Perú: Universidad Nacional de Trujillo, Facultad de Odontología; 2017. Disponible en: http://dspace.unitru.edu.pe/handle/UNITRU/7996.
31. Fujita A, Borges K, Correia R, Gombossy de Melo Franco BD, Genovese MI. Impact of spouted bed drying on bioactive compounds, antimicrobial and antioxidant activities of commercial frozen pulp of camu-camu (Myrciaria dubia Mc. Vaugh). Food Res Int. 2013;54:495-500. doi: https://doi.org/10.1016/j.foodres.2013.07.025.
32. Fujita A, Sarkar D, Wu S, Kennelly E, Shetty K, Genovese MI. Evaluation of phenolic-linked bioactives of camu-camu (Myrciaria dubia Mc. Vaugh) for antihyperglycemia, antihypertension, antimicrobial properties and cellular rejuvenation. Food Res Int. 2015 Nov;77(2):194-203. doi: https://doi.org/10.1016/j.foodres.2015.07.009.
33. Camere-Colarossi R, Ulloa-Urizar G, Medina-Flores D, Caballero-García S, Mayta-Tovalino F, del Valle-Mendoza J. Antibacterial activity of Myrciaria dubia (Camu camu) against Streptococcus mutans and Streptococcus sanguinis. Asian Pac J Trop Biomed [Internet]. 2016 Sep [cited 2018 Sep 19];6(9):[about 1 p.]. doi: https://doi.org/10.1016/j.apjtb.2016.07.008.
34. Mori TJ, Ruiz E, Bardales J, Garcia M, Tresierra A, Arévalo L, col. Efecto antimicrobiano de Myrciaria dubia "camu-camu" y Cyperus luzula "piripiri" sobre microorganismos patógenos. Conoc Amaz. 2013;4(1):49-57.
35. Moromi-Nakata H, Perfecto DR, Cadillo EM, Alvarado EC, Espinoza F. Efectividad in vitro e in vivo de un colutorio a base de Myrciaria dubia "camu camu" sobre bacterias de importancia oral. Theorema (Lima, Segunda época, En línea) [Internet]. 2016 [citado 19 de setiembre de 2018];(1):[1 página]. Disponible en: http://revistasinvestigacion.unmsm.edu.pe/index.php/Theo/article/view/11941.
36. De Azevêdo, JCS, Fujita A, de Oliveira EL, Genovese MI, Correia RTP. Dried camu-camu (Myrciaria dubia HBK McVaugh) industrial residue: A bioactive-rich Amazonian powder with functional attributes. Food Res Int. 2014;62:934-40. doi: https://doi.org/10.1016/j.foodres.2014.05.018.
37. Shringeri PI, Fareed N, Battur H, Khanagar S. Role of probiotics in the treatment and prevention of oral malodor/halitosis: A systematic review. J Indian Assoc Public Health Dent. 2019;17(2):92-6. doi: https://doi.org/10.4103/jiaphd.jiaphd_171_18.
38. Dadashi M, Nasiri MJ, Fallah F, Owlia P, Hajikhani B, Emaneini M, et al. Methicillin-resistant Staphylococcus aureus (MRSA) in Iran: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2018;12:96-103. doi: https://doi.org/10.1016/j.jgar.2017.09.006.
39. Dludla PV, Nkambule BB, Dias SC, Johnson R. Cardioprotective potential of N-acetyl cysteine against hyperglycaemia-induced oxidative damage: a protocol for a systematic review. Syst Rev. 2017;6:96. doi: 10.1186/s13643-017-0493-8.
40. López-Mata AE. Efecto antibacteriano del zumo de Myrciaria dubia, Citrus grandes y Citrus reticula sobre Escherichia coli y Salmonella tiphy. CIENTIFI-K. 2017;5(1):87-92. doi: dx.doi.org/10.18050/Cientifi-k.v5n1a10.2017.
41. Flores D. Uso histórico: Camu camu, Myrciaria dubia (H.B.K) Mc Vaugh [Internet] 2010. Disponible en: http://repositorio.promperu.gob.pe/repositorio/bitstream/handle/123456789/1367/Uso_historico_camu_camu_2010_keyword_principal.pdf?sequence=1&isAllowed=y.
42. Pinedo M, Delgado C, Farroñay R, Del Castillo D, Iman S, col. Camu-camu (Myrciaria dubia, Myrtaceae). Aportes para su aprovechamiento sostenible en la Amazonia Peruana. Lima: Instituto de Investigaciones de la Amazonia Peruana; 2011. Disponible en: http://repositorio.iiap.org.pe/bitstream/IIAP/120/2/Pinedo_Libro_2011.pdf.
43. Arellano-Acuña E, Rojas-Zavaleta I, Paucar-Menacho LM. Camu-camu (Myrciaria dubia): Fruta tropical de excelentes propiedades funcionales que ayudan a mejorar la calidad de vida. Sci Agropecu. 2016;7(4):433-43. doi: http://dx.doi.org/10.17268/sci.agropecu.2016.04.08.
44. Hurtado-Camarena A, Bojórquez-Anaya Y, Montaño-Pérez ML, López-Mendoza JA. Bacterias asociadas a enfermedades periodontales. Oral. 2016;17(54):1374-8.
45. Wang W, Tao R, Tong Z, Ding Y, Kuang R, Zhai S, et al. Effect of a novel antimicrobial peptide chrysophsin-1 on oral pathogens and Streptococcus mutans biofilms. Peptides. 2012;33:212–9. doi: https://doi.org/10.1016/j.peptides.2012.01.006.
46. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745-59. doi: https://doi.org/10.1038/s41579-018-0089-x.
47. Caillet S, Côté J, Sylvain JF, Lacroix M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Control. 2012 Feb;23(2):419-28. doi: 10.1016/j.foodcont.2011.08.010.
48. Mandal SM, Dias RO, Franco OL. Phenolic compounds in antimicrobial therapy. J Med Food. 2017;20(10):1031-8. doi: https://doi.org/10.1089/ jmf.2017.0017.
49. Varela-López A, Bullón P, Giampieri F, Quiles JL. Non-nutrient, naturally occurring phenolic compounds with antioxidant activity for the prevention and treatment of periodontal diseases. Antioxidants. 2015;4(3):447-81. doi: 10.3390/antiox4030447.
50. Weber MT, Hannig M, Pötschke S, Höhne F, Hannig C. Application of plant extracts for the prevention of dental erosion: An in situ/in vitro Study. Caries Res. 2015;49(5):477-87. doi: https://doi.org/10.1159/000431294.
51. Quideau S, Deffieux D, Douat-Casassus C, Pouysengu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. 2011 Jan;50(3):586-621. doi: https://doi.org/10.1002/anie.201000044.
52. Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23(2):174-81. doi: https://doi.org/10.1016/j. copbio.2011.08.007.
53. Basu A, Masek E, Ebersole JL. Dietary polyphenols and Periodontitis-A Mini-Review of Literature. Molecules. 2018 Jul;23(7):1786. doi: https://doi.org/10.3390/molecules23071786.
54. Pal Singh I, Bharate SB. Phloroglucinol compounds of natural origin. Nat Prod Rep. 2006;23(4):558-91. doi: 10.1039/b600518g.
55. Rahman MM, Shiu WKP, Gibbons S, Malkinson JP. Total synthesis of acylphloroglucinols and their antibacterial activities against clinical isolates of multi-drug resistant (MDR) and methicillin-resistant strains of Staphylococcus aureus. Eur J Med Chem. 2018 Jul;155:255-62. doi: https://doi.org/10.1016/j.ejmech.2018.05.038.
56. Akter MS, Oh S, Eun JB, Ahmed M. Nutritional compositions and health promoting phytochemicals of camu-camu (Myrciaria dubia) fruit: A review. Food Res Int. 2011;44(7):1728–32. doi: https://doi.org/10.1016/j.foodres.2011.03.045.
57. Langley PC, Pergolizzi JV Jr, Taylor R Jr, Ridgway C. Antioxidant and associated capacities of camu camu (Myrciaria dubia): a systematic review. J Altern Complement Med. 2015;21(1):8-14. doi: https://doi.org/10.1089/acm.2014.0130.
Correspondence to: Pardo-Aldave Karina
Address. Av. Los Halcones 520. Bellavista, Callao. Perú.
Phone: 01-14510225, 9973-10819
Email:
khiezay@yahoo.es,
infokarina@gmail.com
Received: 06/02/2019
Approved: 04/09/2019
Online: 20/11/2019