|
KEY MESSAGES
|
|
Motivation for the study:
The
use of monoclonal antibodies improves the specificity of antigen detection
techniques in neurocysticercosis, but few monoclonal antibodies actually
exist to improve diagnosis.
Main findings:
The use of magnetic particles
coupled to L protein allows the purification of IgM-type monoclonal
antibodies by applying magnetic force, this way eliminating the need for
centrifugation or precipitation, thus decreasing the quantity of handling
steps, which prevents early denaturation and precipitation.
Implications:
The optimization of a simple platform based on magnetic
particles and L protein allowed the concentration and purification of IgM
antibodies from hybridoma supernatant. This platform can be adapted for
laboratories that do not have specialized infrastructure, since it does not
require the use of columns or ultracentrifuges.
|
Immunoglobulin purification and concentration
procedures are mainly developed for IgG (8-10). However, these methods
may not be as effective for IgM purification. Due to its large size, IgM (~950
kDa) is more susceptible than IgG (~150 kDa) to denaturation and precipitation
by changes in temperature, pH and conductivity, even at low concentrations (11).
Protein A and protein G have been used for a long
time in the purification of antibodies, mainly IgG type that have the constant
fraction (Fc) exposed since it’s the one they use for interaction (12).
However, neither of these two proteins can be used for IgM purification,
because in this antibody, the Fc is hidden (12-13). Unlike proteins A/G,
the L protein (LP) binds to the variable domain of the kappa light chain which
is exposed in all antibody isotypes without interfering with the antigen
binding site. LP offers the additional advantage of not reacting with bovine
and caprine IgG which are generally present in serum-enriched cell culture
supernatants of these animals (14-15).
The use of magnetic particles (MP) can replace the
pre-concentration steps, decreasing antibody handling. Likewise, antibody
binding occurs in solution, and not on a static surface as in chromatographic
columns, providing a 3D interaction between IgM and LP. Moreover, it allows the
separation of purified IgM using a magnetic force or a magnet without the need
for centrifugation or precipitation which makes this method applicable in both
small-scale and large-scale purification, in laboratories that do not have
specialized infrastructure or using automated platforms.
In this study, the use of LP-coupled MP (MP-LP) was
evaluated in the concentration and purification of anti-Taenia solium mIgM.
THE STUDY
Two mIgM clones (TsW5 and TsW8) specific to T. solium cyst
proteins were used. The production and maintenance of hybridomas were performed
according to the protocols described above (5). Briefly, cell cultures of
hybridomas were maintained in advanced DMEM medium (Dulbecco’s modified Eagle’s
medium, Thermo Fisher, USA) supplemented with 10% fetal bovine HyClone
serum (Thermo Fisher, USA), at 37 ºC in 5% CO2. Hybridoma supernatants (HS)
were collected after one to two weeks of incubation, filtered with 0.22 µm
nitrocellulose filters and stored at 4 ºC for up to four days.
Evaluation of HS concentration methods
Three
methods of HS concentration were evaluated: pressure filtration (Amicon®
Stirred Cells), passive ultrafiltration (Minicon® Concentrator) and
ultrafiltration by centrifugation (Amicon® Ultra Centrifugal Filters), all
filters with a minimum cut-off of 10 kDa (all from Merk Millipore, USA). The
protein concentration of the supernatants was determined using the Bradford
method (Bradford Protein Assays, Thermo Scientific, USA), before and after
being concentrated; the concentration factor was calculated by dividing the
protein concentration of the concentrated supernatant with the protein
concentration of the initial supernatant. An equal volume of initial
supernatant was evaluated in all three concentration methods. Since the protein
concentration will mainly reflect the presence of albumin and other proteins,
the recovery percentage (RP) in terms of mIgM activity was also calculated and
measured by ELISA test using a crude extract of T. solium cysts as
antigen, and a secondary peroxidase-labelled antibody to mouse IgM produced in
goat (Peroxidase-Labeled Antibody To Mouse IgM, Produced in Goat, KPL
Laboratories, USA) (5). Optical density (OD) was measured at 590 nm using the
Spectra-Max-340 (Molecular Devices, Sunnyvale, California, USA). The RP of mIgM
was calculated by dividing the optical density of the concentrated supernatant
by the optical density of the initial supernatant multiplied by 100.
Purification of mIgM
Superparamagnetic
particles (0.5 μm of mean radius) coupled with recombinant LP (PierceTM Protein
L Magnetic Beads, Thermo Scientific, USA) were used. To determine which
concentration of LP-MP recovers more than 90% mIgM in HS, five different HS
volumes were incubated (100 μl, 250 μl, 500 μl, 750 μl and 1500 μl) with 400 μg
of LP-MP in a total volume of 1500 μl of Tris-buffered saline (TBS, Tris 25
mM, NaCl 150 mM, pH 7.4). A volume of 100 μl of HS was used as a reference,
since the ELISA test showed that no mIgM was left in the HS after incubation
with LP-MP, i.e., all mIgM present in the medium was captured. Incubation of HS
and LP-MP was performed for 45 minutes, at room temperature (RT) and under
constant agitation (500 rpm, LabRoller). After incubation, the mIgM + PM-PL
compounds were separated from the medium using a magnetic holder (DynaMagTM-2
Magnet, Thermo Scientific, USA) for one minute. For the separation of mIgM from
PM-PL, 2M glycine, pH 2.0 at ambient-temperature, was used with constant
agitation for 15, 45 and 60 minutes (Figure 1). The mIgM solution was
neutralized with 1M Tris, pH 8.5, then the buffer exchange to
phosphate-buffered saline was performed. The purified mIgM was concentrated by
ultrafiltration by centrifugation (10 kDa cut).
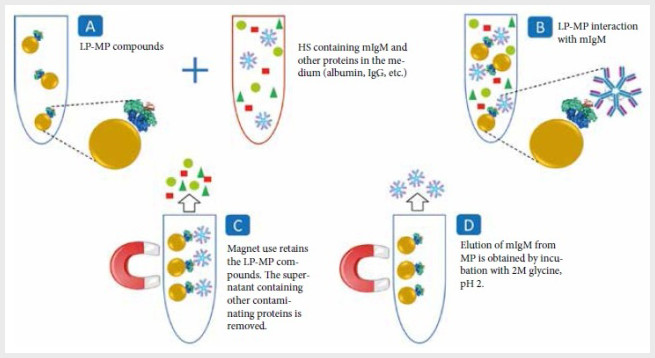
Figure 1. Schematic representation of the mIgM
monoclonal antibody purification using magnetic particles coupled to L-protein
(figure developed by the authors). A) and B) Monoclonal IgM antibodies (mIgM)
present in the hybridoma supernatant (HS) will bind in solution to the
L-protein (LP) coupled to the magnetic particles (LP-MP). C) The magnetic
particles (MP) are separated from the HS with the help of a magnetic holder. D)
The purified mIgMs are eluted from the LP-MP using an acidic glycine solution.
Purity evaluation of
eluates
To
evaluate purity of mIgM, sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) was performed under reducing conditions using 10%
polyacrylamide gel and Coomassie blue stain.
RESULTS
In regard to protein concentration, the highest concentration
factor was obtained when supernatants were concentrated using pressure
filtration (4.4 times), followed by passive ultrafiltration (3.9 times) and
ultrafiltration by centrifugation (2.5 times). In terms of mIgM activity
measured by ELISA test, all three methodologies produced a RP above 80%
(pressure filtration: 92.5%95%, passive ultrafiltration: 84.2%-93.9% and
ultrafiltration with centrifugation: 82.1%-84.3%). However, protein precipitation
was observed twice using pressure filtration, for this reason, to minimize mIgM
precipitation and due to the lower cost and shorter time needed for
concentration (15 minutes), compared to the other methods (~12 hours for
passive ultrafiltration or ~5 hours for pressure filtration), it was decided to
use ultrafiltration by centrifugation for future analyses.
The optimal ratio of µg LP-MP to µl HS was evaluated
using the ratio of 400 µg LP-MP to 100 µl HS as a reference. The percentage of
functionally active mIgM that were not captured or eluted by PM-PL was
calculated by subtracting the OD value of HS after incubation with LP-MP minus
the OD value of HS at the reference ratio, multiplied by 100. It was found that
the lowest percentage loss was obtained using 400 µg LP-MP with 250 µl HS
(0.6%) and the highest RP was obtained using 500 µl HS (99.3%) (Table). It is
concluded that the best way to recover mIgM is when the ratio of µg of LP-MP to
µl of HS is 0.8.
Table 1. Optimization of the monoclonal IgM antibody
purification protocol from hybridoma culture supernatants using magnetic
particles coupled to L-protein
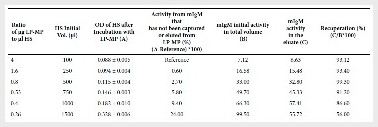
400 μg of LP-MP were added to the HS in a final volume of 1500 μl
of Tris-buffered saline solution as a binding buffer Antibody activity was
measured by ELISA test to determine the ability of the antibody to recognize
sonic cysts of T. solium LP-MP: L-protein coupled magnetic particles; HS: hybridomas
supernatant; OD: optical density measured at 590 nm
To determine if a pre-concentration step is needed
before incubation of HS with LP-MP, we compared the RP obtained with 50 μl of
concentrated HS (initial volume 2500 μl, concentration factor 50x) and 2500 μl
of unconcentrated HS, obtaining a RP of 95.46% (coefficient of variation, CV
0.77%) and 96.93% (CV 0.43%) when a concentration and non-concentration step
was carried out, respectively, which indicates that a pre-concentration stage
is not required.
In this study, a glycine buffer, pH 2.0, was used for
the separation of mIgM from LP-MP. According to the manufacturer’s
recommendations, the elution or separation time with acidic solution should not
exceed 15 minutes to prevent degradation of the antibody. Considering the
activity of the antibody, we observe that the elution time of 15 minutes has a
RP of 78.5%. However, when the time increases to 45 minutes the RP increases to
94.1%. Conversely, if the time is increased to 60 minutes, the RP decreases to
83%, probably due to some degree of denaturation caused by the pH.
In the electrophoresis results, different proteins,
mainly albumin (67 kDa), were observed before purification (Figure 2). After
the LP-MP purification process, only two bands of 73 kDa and 25 kDa,
corresponding to the heavy and light chains, respectively, of IgM, were
observed, confirming the purity of the eluates.
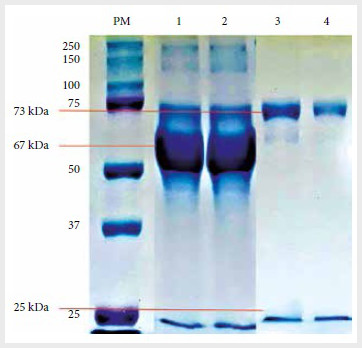
Figure 2. Coomassie blue staining after polyacrylamide
gel electrophoresis with 10% sodium dodecyl sulfate of monoclonal IgM
antibodies (TsW5 and TsW8) before and after purification with L protein coupled
magnetic particles.
The number of times that LP-MP can be re-used for
antibody purification using the same mIgM clone was also evaluated. A RP of
81.9% was observed after reusing the same batch of LP-MP 21 times.
DISCUSSION
It is shown that magnetic particles can be used to concentrate IgM
from a solution in a single step (16), reducing antibody
manipulation that can lead to degradation and denaturation. When evaluating
pre-concentration methods, protein precipitation was observed twice using
pressure filtration, possibly due to the mechanical forces and temperature rise
that led to IgM desolubilization. This is an advantage of MP over the use of
chromatographic columns, since with the use of columns, preconcentration is
necessary, probably because the attraction and frequency of interaction
opportunities are lower compared to when using particles (17-18),
and also to avoid passing several times the culture through the column,
especially when it is higher than 1 ml volume, which can be tedious. The use of
magnetic particles favors the interaction of mIgM and LP in solution in a
single step, and also prevents the loss of mIgM immunogenic activity, since
this can occur during the binding of IgM to other resins (16).
Based on the results of electrophoresis under reducing conditions, it was shown
that the use of L-protein produces a highly purified IgM eluate without IgG
contamination present in bovine serum that is used as a supplement in cell
culture (14).
A longer mIgM elution time than recommended by the
manufacturer was needed (45 minutes vs. 15 minutes), probably due to the high
affinity between IgM and LP (Ka = 1010 M- 1) (19). Other studies have reported
that IgM is able to withstand exposure to acidic pH (20). In
our study, the short handling time (two hours total) compared to classical
affinity chromatography methods (ten hours to two days) may have contributed
to the lower risk of denaturation of mIgM and increased the likelihood of
resistance to acidic elution (12). Our RP (> 90%) is higher than
previously reported using MP coupled with anti-IgM antibodies (70%), probably
due to the higher affinity of LP to IgM (17).
We present the results of concentration optimization
and mIgM purification from hybridoma supernatant using a simple platform based
on the use of magnetic particles and LP. This methodology can be adapted to non-specialized
protein concentration laboratories since it does not require the use of
columns or ultracentrifuges. It was demonstrated that HS does not need to be
concentrated. Therefore, if the use of LP-MP is to be implemented on a medium
or large scale, it is necessary to have magnetic supports for 50 ml tubes. The
average time for the separation of LP-MP from the medium should be between
three to five minutes, in order to ensure the total recovery of MP. It is
demonstrated that incubation of HS and LP-MP can be carried out at
ambient-temperature or 4 °C. However, the incubation should be at 4 °C to avoid
degradation of the analyte in the sample.
Acknowledgements:
We
thank Dr. Cristina Guerra and Dr. Miguel Orrego for facilitating access to
hybridoma supernatants. To Adriana Paredes, for her guidance in the initial
steps of the study. To Helena Jahuira, and Giuliana Oyola Lozada for
coordinating the purification procedures. To Heydi Toro for the administrative
and financial coordination of the project. To Doctors Patricia Sheen, Manuela
Verastegui, and José Espinoza for providing us with access to their laboratory
equipment. To Dr. Theodore Nash Sukwan Handali for corrections and suggestions
in writing the article.
REFERENCES
1. Del Brutto OH, Garcia HH. Neurocysticercosis. Handb Clin Neurol.
2013;114:313–25. doi:
10.1016/B978-0-444-53490-3.00025-X.
2. Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic
review of the frequency of neurocysticercosis with a focus on
people with epilepsy. PLoS Negl Trop Dis.
2010;4(11):e870. doi:
10.1371/journal.pntd.0000870.
3. Garcia HH, Nash
TE, Del Brutto OH. Clinical
symptoms, diagnosis, and treatment
of neurocysticercosis. Lancet
Neurol. 2014;13(12):1202-15.
doi:
10.1016/S1474-4422(14)70094-8.
4. Parija M, Biswas R, Harish BN, Parija SC. Detection of specific cysticercus antigen in the urine for diagnosis of neurocysticercosis. Acta Trop.
2004;92(3):253-60. doi: 10.1016/j.actatropica.2004.08.007.
5. Paredes A, Sáenz P, Marzal MW, Orrego MA,
Castillo Y, Rivera A, et al. Anti-Taenia solium monoclonal antibodies for the detection
of parasite antigens in body
fluids from patients with neurocysticercosis.
Exp Parasitol. 2016;166:37–43. doi:
10.1016/j.exppara.2016.03.025.
6. Rodriguez S, Wilkins P, Dorny P. Immunological and molecular diagnosis of cysticercosis. Pathog Glob Health. 2012;106(5):286–98. doi:
10.1179/2047773212Y.0000000048.
7. Knutson VP, Buck RA, Moreno RM. Purification
of a murine monoclonal antibody
of the IgM class. J Immunol Methods. 1991;136(2):151–7. doi: 10.1016/0022-1759(91)90001-V.
8. Walker ID. Detection,
Purification, and Utilization
of Murine Monoclonal IgM Antibodies. In: Monoclonal Antibody
Protocols [Internet]. Humana Press;
1995 [citado el 2 de junio del 2018]. Disponible en:
https://link.springer.com/protocol/10.1385/0-89603-308-2:183.
9. Rigi G, Ghaedmohammadi S, Ahmadian G. A comprehensive review on staphylococcal protein A (SpA): Its production and applications. Biotechnol Appl Biochem. 2019;66(3):454-464. doi:
10.1002/bab.1742.
10. Li Y. A brief introduction of IgG-like bispecific antibody purification: Methods for removing product-related
impurities. Protein Expr Purif. 2019;155:112–9. doi:
10.1016/j.pep.2018.11.011.
11. Gagnon P, Hensel F, Andrews P, Richieri R. Recent advances in the purification of IgM monoclonal antibodies. En:
3rd Wilbio Conference on Purification of Biological Products Waltham. Massachussetts; 2007.
12. Hober S, Nord
K, Linhult M. Protein A chromatography for antibody purification. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848(1):40–7.
doi:
10.1016/j.jchromb.2006.09.030.
13. Schroeder HW, Cavacini
L. Structure and Function
of Immunoglobulins. J Allergy
Clin Immunol. 2010;125(202):S41–52. doi:
10.1016/j. jaci.2009.09.046.
14. Nilson BH, Lögdberg
L, Kastern W, Björck L, Åkerström B. Purification of antibodies using protein L-binding framework structures in the light chain variable domain. J Immunol Methods. 1993;164(1):33–40. doi: 10.1016/0022-1759(93)90273-A.
15. Paloni M, Cavallotti C. Molecular Modeling
of the Interaction of Protein L with Antibodies. ACS Omega. 2017;2(10):6464–72.
doi: 10.1021/
acsomega.7b01123.
16. Castro-Sesquen
YE, Kim C, Gilman RH, Sullivan DJ, Searson PC. Nanoparticle-Based Histidine-Rich Protein-2 Assay for the Detection
of the Malaria Parasite Plasmodium
falciparum. Am J Trop Med Hyg. 2016;95(2):354–357.
doi: 10.4269/ajtmh.15-0772.
17. Quitadamo IJ, Schelling ME. Efficient purification of mouse anti-FGF receptor IgM
monoclonal antibody by magnetic beads. Hybridoma. 1998;17(2):199–207. doi: 10.1089/hyb.1998.17.199.
18. Karlsson GB, Platt FM. Analysis and isolation of human transferrin
receptor using the OKT-9
monoclonal antibody covalently
crosslinked to magnetic beads. Anal Biochem. 1991;199(2):219-22. doi:
10.1016/0003-2697(91)90093-9.
19. Gautam S, Loh K-C. Immunoglobulin-M purification--challenges and perspectives. Biotechnol Adv. 2011;29(6):840–9. doi: 10.1016/j.
biotechadv.2011.07.001.
20. Mueller M, Wan C, Hoi KM, Kim DY, Gan HT, Bardor M, et al. Immunoglobulins M survive low-pH conditions used for virus inactivation and for elution from bioaffinity
columns. J Pharm Sci. 2013;102(3):1125–32. doi: 10.1002/jps.23428.
Funding sources:
This study was funded by the National Council for
Science, Technology, and Technological Innovation (FONDECYT - CIENCIA ACTIVA)
[115-2015-FONDECYT-DE] and the NIH/Fogarty Training Grant [TW001140].
Citation:
Perez LA, Castillo Y, Espinoza C, Toribio LM, Santos
Y, Martel KS, et al. Use of magnetic particles in the purification of
IgM antibodies against Taenia solium. Rev Peru Med Exp Salud Publica. 2020;37(1):104-9. doi: https://doi. org/10.17843/rpmesp.2020.371.4628
Correspondence to:
Yagahira Castro-Sesquen; 13229 Orsay St, Clarksburg, Maryland,
Estados Unidos;
yagahiraelizabeth@hotmail.com.
Authorship contributions: LAP and YCS participated in the
conception, design and writing of the article, in the analysis and
interpretation of data, and in the final approval of the version for
publication. HHG, JAB, PPW, YC participated in the design and critical review
of the manuscript, and in the final approval of the version for publication.
CE, LMT, YS, KSM participated in collection of data, in the critical review of
the manuscript, and in the final approval of the version for publication. All
authors are responsible for all aspects of the manuscript, to ensure that
issues regarding the accuracy or completeness of any part of the manuscript
will be properly investigated and resolved.
Conflicts of interest:
All authors have none to declare.
Received:
27/06/2019
Approved:
11/12/2019
Online:
19/03/2020


