Maritza D. Placencia-Medina
Tomás Y. Miranda-Tomasevich
Miriam Moreno-Hinojosa
Mónica G. Retuerto-Figueroa
10.17843/rpmesp.2020.373.4817
ORIGINAL ARTICLE
In vitro cytotoxic and genotoxic effect of the crude and ethanolic extract from the rhizome of Curcuma longa L.
Martha F. Cosquillo-Rafael
![]() , Bachelor in Pharmacy and Biochemistry
, Bachelor in Pharmacy and Biochemistry
Maritza D. Placencia-Medina
![]() , Doctor of Pharmacy and Biochemistry
, Doctor of Pharmacy and Biochemistry
Tomás Y. Miranda-Tomasevich
![]() , Bachelor in Biology
, Bachelor in Biology
Miriam Moreno-Hinojosa
![]() , Master of Public Health
, Master of Public Health
Mónica G. Retuerto-Figueroa
![]() , Master in
Environment and Sustainable Development
, Master in
Environment and Sustainable Development
ABSTRACT
Objectives: To determine the in vitro cytotoxic and genotoxic effect of the crude and ethanolic extract from the Curcuma longa L. rhizome.
Materials and methods: The cytotoxic effect was evaluated using DU-145, HT-29, 3T3 BALB/c cell lines. The growth percentages in 48 hours; and the half maximal inhibitory concentration (IC50) were determined. The genotoxic effect on human genomic DNA was determined using the Tomasevich method.
Results: Crude extract produced an IC50 of 12.98 ± 0.21 μg/mL for the HT-29 tumor cell line, which is lower than the value obtained for DU-145, with an IC50 of 36.77 ± 9.12 μg/mL. The ethanolic extract presented an IC50 of 13.24 ± 0.77 and 20.54 ± 2.58 μg/mL for both cell lines, respectively; the curcumin standard compound presented an IC50 of 3.96 ± 0.60 and 13.94 ± 2.79 μg/mL, respectively. Crude extract concentrations of 50 and 100 mg/mL fragmented between 40% to 95% of human genomic DNA; while at 200 mg/mL, fragmentation was greater than 95%. The ethanolic extract at all concentrations did not fragment the DNA. Curcumin at 200 mg/mL fragmented less than 5% of human genomic DNA.
Conclusions: The crude and ethanolic extracts of Curcuma longa L. demonstrate different in vitro cytotoxic effects for the human tumor cell lines DU-145 and HT-29; similar to the standard curcumin compound. The crude extract of Curcuma longa L. shows a potent genotoxic in vitro activity against human genomic DNA; this type of effect is not produced by the ethanolic extract.
Keywords: Genotoxic; Cytotoxins Agents; Curcumine, Genomic DNA; Cell Line; HT29 Cells; BALB 3T3 Cells; Gel Electrophoresis (Source: MeSH NLM).
INTRODUCTION
Cancer is a public health problem in Peru. During
2017, 10,650 cancer cases were registered; of which 7,537 (70.8%) were new as
per the consolidated figures from 47 national health establishments (1).
Cancer incidence is 20% higher in men than in women, while the cancer mortality
rate is 40% higher (2). Colon cancer therapy in men and women, as
well as prostate cancer, is considered to have a high economic impact.
In the human body, chronic inflammation can affect
homeostasis and metabolism of normal cells, causing susceptibility to genomic
instability, which can lead to uncontrolled cell growth and tumorigenesis (3,4). During this inflammation process, a variety of
cytotoxic mediators are produced, such as free radicals, reactive oxygen
species (ROS) and reactive nitrogen species (RNS), which play an important role
in the damage to deoxyribonucleic acid (DNA) (5).
Several studies established the preventive effect of
consuming fruits, vegetables, spices, and aromatic herbs. These products have
bioactive phytochemicals that have anticarcinogenic, antimutagenic (6,7),
and antioxidant properties; which prevent, neutralize, or repair in a direct or
indirect way the cellular damage caused by free radicals, such as the oxidation
to lipids, proteins, and nucleic acids (8).
The turmeric, Curcuma longa L., is a spice used
in traditional Chinese medicine to treat inflammatory conditions (9).
According to the Indian Ayurvedic pharmacopoeia and the Chinese pharmacopoeia,
it is used as a tonic, a stomach carminative, and may relieve pain, eliminate
blood stasis, and stimulate menstrual flow. The main polyphenolic phytochemical
constituents of Curcuma longa L. include three curcuminoids
(curcumin, demethoxycurcumin, and
bisdemethoxycurcumin), whose main component, polyphenol curcumin, has great
antioxidant capacity (10). The crude (CE) and ethanolic (EE) 96%
extracts of Curcuma longa L. rhizome have in vitro antioxidant
capacity determined by DPPH (2,2-diphenyl-1-picrylhydrazil) and ABTS
(2,2-Azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid) methods (11,12).
Also, polyphenol curcumin has anti-inflammatory and anti-cancer properties,
modulating the epigenetic alterations typically associated with cancer (13).
Genotoxicity is the ability to trigger damage in a
part or in the whole genetic material of a cell, ultimately on the DNA molecule
(14,15). There are experimental investigations
on genotoxic potential about curcumin isolated from Curcuma longa L.
that do not report cytotoxic or genotoxic potential (16), and others
where the supplementation of this isolated principle significantly antagonizes
genotoxic effects (17).
Therefore, the aim of this research was to determine
the cytotoxic and genotoxic in vitro effect of the crude and ethanolic
extract of Curcuma longa L.’s rhizome.
|
KEY MESSAGES |
|
Motivation for the study: It is necessary to look for alternative cancer treatments. Some
people use the crude extract of Curcuma longa L. as an alternative
treatment for colon and prostate cancer. Main findings: The crude and ethanolic extract of Curcuma
longa L. has differentiated cytotoxic activity for prostate and colon
cancer cell lines. The crude extract and curcumin were found to be genotoxic,
and the ethanolic extract, non-genotoxic. Implications: The use of the ethanolic extract of Curcuma
longa L. could be an alternative for developing an affordable phytomedicine for the treatment of colon and prostate
cancers. |
MATERIALS AND METHODS
This is a quantitative, analytical study with an
experimental design. The experimental units were cells belonging to the human
cell lines DU-145 (prostate carcinoma), HT-29 (colon adenocarcinoma), and 3T3
(normal mouse fibroblasts) provided by the Research and Development Laboratory
of the Universidad Peruana Cayetano Heredia (LID-UPCH). The human genomic DNA
was provided by the Research Center for Molecular Biology and Bioinformatics of
the Universidad Nacional de San Cristobal de Huamanga.
The samples of the complete specimen and rhizomes of Curcuma longa L.
were collected in the province of Chanchamayo, Junin, Peru. The taxonomic identification was carried out
by a taxonomist/curator from the Universidad de la Amazonía.
Obtention of raw extract
The fresh, clean, peeled and weighed rhizome was
processed in a Philips brand extractor, and the CE was obtained. It was
evaporated to dryness in a stove with circulating air at 40 °C. The dry
extract obtained was stored in a suitable container, a labeled amber bottle, to
protect it from light and humidity.
Obtention of ethanolic extract
The rhizome was peeled and dried in an oven with
circulating air (Memmert) at 40 °C. Afterwards, it
was milled in a Willey Mill grinder with Arthur H. Thomas CO blades, and a dry
and homogeneous powder was obtained. It was then weighed and mixed with 96%
ethanol in a (2:1) ratio of solvent: dry rhizome powder, in an amber flask; it
was then macerated for 28 days at room temperature with rotary movements for 15
minutes each day; it was filtered with gauze layers and the filtered solution
was evaporated to dryness in an oven at 40 °C.
Evaluation of cytotoxic activity
The experiment was developed in the Cell Biology and
Virology Laboratory of the LID-UPCH. The DU-145 cell line was cultured and
maintained in Minimum Essential Medium (MEM) culture medium supplemented with
10% fetal bovine serum and 50 μg/mL gentamicin; the
HT-29 cell line, in Roswell Park Memorial Institute (RPMI)-1640 culture medium
supplemented with 7.5% fetal bovine serum and 50 μg/mL
gentamicin; and the 3T3 BALB/c cell line, in Dulbecco’s Modified Eagle medium
(DMEM) culture medium supplemented with 10% fetal bovine serum and 50 μg/mL gentamicin.
To activate each cell line, the cell monolayer was washed
twice with 5 mL Ca- and Mg-free Hanks solution, then 1 mL of the
trypsin-ethylenediaminetetraacetic acid (EDTA) solution was added and removed
after 10 seconds. It was then incubated for 10 minutes at 37 °C, after
which the activated cells were suspended with 3 mL of the corresponding culture
medium.
The cells were inoculated into 96-well cell culture
plates and incubated at 37 °C in a humid atmosphere of 5% CO2
and 95% air for 24 hours to fix the cells in the plate wells. Each plate
containing each of the cell lines was fixed in situ with trichloroacetic
acid (TCA) to obtain the cell values at zero-time before adding the extracts.
Then, each well received 40 μL
of each serial dilution at increasing concentrations from 3.9 μg/mL to 62.5 μg/mL of the CE
and EE from Curcuma longa L. and curcumin, and from 0.03 μg/mL to 1.95 μg/mL of 5‑fluorouracil
(5-FU); the zero plate wells received 40 μL from
MEM. It was homogenized with Heidolph plate agitator
for 30 seconds and incubated for an additional 48 hours under the same
conditions. Then, 100 μl of 20% TCA were added
to stop the assay and it was refrigerated at 4 °C for 1 hour. The TCA was removed,
and each plate was washed 5 times with 500 mL of water, drained and dried. The
anti-tumor drug 5-FU was used as a positive control.
Sulforhodamine B assay
Determination of cell growth inhibition was performed
by the sulforhodamine B (SRB) cytotoxicity assay
described by Skehan et al. (18),
which allows to indirectly estimate the number of
viable cells, since the SRB dye can stain the total cell protein.
Then, 50 μl of the 0.2% SRB
solution in 1% acetic acid were added to the cells fixed with TCA in each well
and left to stand at room temperature for 30 minutes. The excess of non-protein
bound SRB dye was removed by rapid washing for 5 times with 1% acetic acid,
then the culture plates were allowed to dry. The SRB
dye bound to proteins was eluted with a 10 mM solution of Tris base (Tris
hydroxymethyl aminomethane) at a 10.5 pH. Finally,
the optical density was determined, which is directly proportional to the
number of cells, using an Ibo-Rad model 450 to 510 nm wavelength microplates
reader. The anti-tumor drug 5-FU was used as a positive control. The inhibitory
concentration 50 (IC50) is the extract concentration that inhibits
50% of the cell growth. All the assays were carried out in triplicate.
Genotoxic activity evaluation using the “Tomasevich method”
The “Tomasevich method” (19)
is a modification of the “comet assay” (20,21)
and is used to evaluate the in vitro genotoxic effect of medicinal
plants and their extracts or phytotherapeutic
products against genomic DNA. After an incubation period, the fragmentation degree
of the DNA strands is measured by subjecting it to agarose gel electrophoresis
and staining it with ethidium bromide in order to visualize it in an ultra-violet (UV) light transilluminator and to record
images with a digital camera (19).
For this purpose, we had a stock of human genomic DNA
at a concentration of 1,500 ng/µL in a final volume of 200 µL for each test,
then we proceeded to prepare the solutions of Curcuma longa L. rhizome
extract at concentrations of 5, 10, 25, 50, 100 and 200 mg/mL, respectively,
using sterile bi-distilled water as solvent. A battery of nine 500 µL tubes was
labeled with numbers (1 to 9) and the assay components were discharged as
indicated in Table 1, immediately incubated at 37 °C for one hour to
enable the action of the extract on the genomic DNA. It should be noted that
the preparation of the extract at different concentrations was independent for
each assay, i.e. with CE, EE, and curcumin (19,22-24).
Table 1. Preparation of compounds for in vitro
genotoxicity testing of crude and ethanolic extract
of Curcuma longa L. rhizome and curcumin, respectively, at
concentrations of 5, 10, 25, 50, 100 and 200 mg/mL against human genomic DNA.
PK: proteinase K;
Target: extract or curcumin, respectively, used as a target; Control: only
human genomic DNA, used as a control; CE: crude extract; EE: ethanolic extract; NI: not included.
After the incubation period, contents from each of the
tubes were seeded into the respective wells of 1% agarose gel immersed in Triz-EDTA (TE) run buffer with a 1X concentration,
installed in the Biometra® brand electrophoresis
chamber. The 10 µL loading volume is the result of 8 µL of the sample plus 2 µL
of run dye (bromophenol blue plus xylene). In the first well, the molecular
size marker of 100 base pairs (bp) was seeded; in the following six wells, the
treated products were seeded with the extracts at their respective
concentrations; the next well contained the extract at the concentration of 100
mg/mL as a target; the next well only contained DNA at the concentration of
1,500 ng/µL as a control; and the last well was seeded with the treatment
product with extract of 100 mg/mL plus the enzyme proteinase K. The electrophoretic
run was performed at 30 volts for 180 minutes (19,22-24).
Once the electrophoretic run was finished, the agarose
gel was removed, then immersed for 15 minutes in 1% ethidium bromide contained
in an exclusive tray, later it was rinsed twice with running water, it was
installed in an Ultra Lum brand UV transilluminator and the photographs were taken
with a 12.1-megapixel full HD Canon 20X brand digital camera to interpret the
results. Each test was repeated four times with each of the different extracts
of Curcuma longa L. and curcumin (19,22-24).
Data analysis
To analyze the in vitro cytotoxic activity, the
data were grouped and presented Excel tables; and the statistical analysis, in SPSS
21. The IC50 was determined through the analysis of linear
regression with a 95% confidence interval. Results are expressed as the mean
and standard deviation of the IC50 values, obtained in triplicate.
The ANOVA test was carried out according to each sample type, comparing the
respective averages in the cell line groups and considering a 95% confidence
interval.
To study the genotoxic effect, numerical values were
assigned to the different degrees of DNA fragmentation, considering the color intensity
of the corresponding lane band, shown in the photographic records. To evaluate
the genotoxicity due to the degree of DNA fragmentation, the non-parametric
Kruskal Wallis test was used, because the data do not show normal distribution.
This test allows us to determine if at least one treatment differs
significantly in its effect from the rest of the treatments.
RESULTS
The growth percentages of the DU-145 cell line varied from
56.7% to 93.6% for CE and from 11% to 104.9% for EE; for the HT-29 cell line,
the variation was from 12.1% to 96.2% for CE and from –0.2% to 79% for EE; and
for the 3Q3 control cell line, the range was from 34.8% to 100% for CE and from
–1.1% to 92.2% for EE. For the 5-FU positive control, the lowest percentage of
growth (18.5%) was observed in the 3Q3 cell line, while the highest percentage
of growth (114.4%) was seen in the DU-145 cell line, shown in Table 2.
Table 2. Inhibitory
concentration 50 of crude and ethanolic extract of Curcuma
longa L. rhizome, curcumin, and 5‑fluorouracil with cytotoxic effect
according to tumor cell lines DU-145 and HT-29 and 3T3.
a Mean and standard deviation; b Anova test.
NP: not performed.
Table 3 shows the IC50 values for the CE
and EE of Curcuma longa L., as well as the 5-FU control in the studied
tumor cell lines. The behavior of the extracts showed differentiated cytotoxic
capacity in all the tumor cell lines.
Table 3. Numerical values of in vitro genotoxicity tests of crude and ethanolic extract of Curcuma longa L. rhizome and curcumin at concentrations of 5, 10, 25, 50, 100 and 200 mg/mL, against human genomic DNA.
0: DNA
Fragmentation < 5%; 1: DNA fragmentation 5 to 20%; 2: DNA fragmentation 20
to 40%; 3: DNA fragmentation 40 to 95%; 4: DNA fragmentation > 95%.
Source: Colins et al. (21)
Figure 3. Kruskal Wallis test to determine the level of in vitro
genotoxicity of the crude (A), ethanolic (B) extract
of Curcuma longa L. rhizome and curcumin (C) at concentrations of 5, 10,
25, 50, 100 and 200 mg/mL, against human genomic DNA at 1500 ng/µL, incubated
at 37 °C for one hour.
The EE of Curcuma longa L. showed a lower
percentage of cell growth in the DU-145, HT-29 and 3T3 cell lines (Figure 1A,
1B and 1C) compared to the CE.
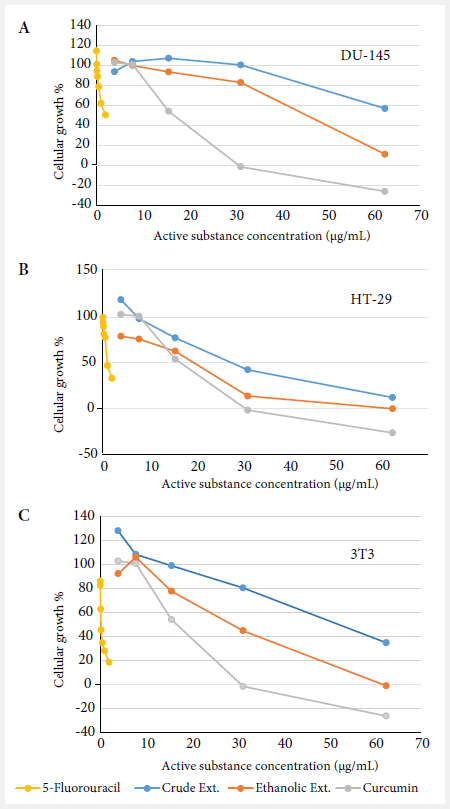
Figure 1. Growth percentage curves of cell lines (A) DU-145, (B) HT-29 and (C)
3T3 at different concentrations of Curcuma longa L., curcumin and 5
fluorouracil extracts.
The photographic records in Figure 2 reveal the
results of the in vitro genotoxicity of CE and EE of the rhizome of Curcuma
longa L. and curcumin against human genomic DNA. CE at a concentration of
25 mg/mL fragmented between 5% and 20% of the DNA; at 50 mg/mL and 100 mg/mL
the fragmentation was 40% to 95% of the DNA, while at a concentration of 200
mg/mL the fragmentation was greater than 95%. EE did not fragment DNA at any of
the tested concentrations from 5 mg/mL to 200 mg/mL, which shows no genotoxic
effect. Meanwhile, at a concentration of 200 mg/mL, curcumin fragmented 5% to
20% of human genomic DNA.
Figure 2. Photographic record of the in vitro genotoxic assay of the crude (A), ethanolic (B) extract of Curcuma longa L. rhizome and
curcumin (C) at concentrations of 5, 10, 25, 50, 100 and 200 mg/mL against
human genomic DNA at 1,500 ng/µL, incubated at 37 °C for one hour. In Figure A,
No. 4 and No. 5 correspond to concentrations of 50 and 100 mg/mL, respectively,
and show DNA fragmentation between 40% and 95%, while No. 6, which corresponds
to 200 mg/mL, shows DNA fragmentation > 95%, all compared to No. 8, which is
the DNA band that received no treatment and serves as a control. In Figure C,
No. 6, which corresponds to 200 mg/mL shows DNA
fragmentation < 5% compared to No. 8.
At a concentration of 100 mg/mL, plus the enzyme
proteinase K, CE fragmented 40% to 95% of human genomic DNA; at 100 mg/mL, plus proteinase K, EE did not fragment DNA; likewise, at 100 mg/mL, plus the enzyme
proteinase K, curcumin did not fragment human genomic DNA (Figure 3).
DISCUSSION
The results found show that the crude and ethanolic
extract of Curcuma longa L. rhizome have cytotoxic effect and potential
anti-tumor activity against HT-29 and DU-145 cells, by revealing a decrease in
cell survival when the concentrations of Curcuma longa L. and curcumin
extracts are increased. We used three concentrations closest to the IC50
to build a line (25).
The cytotoxic activity of the extracts and curcumin is
different between the treated cells. However, the best activity was exhibited
by the ethanolic extract, which presented a lower IC50. The CE and
EE with curcumin inhibited the growth of HT-29 cells more effectively than of
the DU-145 cell line.
Using human murine colon 26 cells, human HT-29 and HCT
116 from colorectal cancer (CRC), Kuete et al.
(26) demonstrated the in vitro cytotoxic activity of CE
(18.8% g/g) in all tests, particularly by finding an IC50 of 15 μg/mL for CRC after been incubated between 48 and 72 hours;
while the IC50 for curcumin was 5 μg/mL. These results are similar to those from our study and
are encouraging because of their cytotoxic effect against HT-29.
Yue et al. (27) conducted an in
vitro study on the cytotoxic activity of the isolated components α/β-turmerone, ar-turmerone, curcumin
and the ethanolic extract of Curcuma longa L, which inhibited the growth
of colon cancer cells at a time-dependent dose. The ethanolic extract had the
highest lC50 at 11.67 μg/mL
in the HT-29 cell line, a similar result to ours. These findings would
provide scientific evidence on the use of turmeric as an adjuvant therapy for
colorectal cancer.
Cao et al. (28) have reported that
curcumin inhibits the growth of HT-29 cells (colon carcinoma) with a IC50 of 40.7 ± 0.5 mΜ
in in vitro culture for 24 hours. This cytotoxic activity seems to be
mediated by the induction of apoptosis in these cells; our results corroborate
this antiproliferative activity that shows therapeutic potential.
Hong et al. (29) state that curcumin
inhibits the cellular proliferation of the DU-145 cell line (prostate cancer)
depending on the dose, starting with 10 g up to 30 g where a
IC50 is obtained, without showing any major effect after 50 g. Our
results behaved in a similar way, indicating an antiproliferative and
antimetastatic effect in the prostatic tissue.
Calaf et al. (30) point out the ability
of curcumin to induce apoptosis in tumor cells through various methods, such as
assays for caspase-3/7 activity, adnexin V, poly (ADPribosa) polymerase-1 activation and caspase-3 protein
expression, nuclear factor (NF) - transcription factor κ B and proliferating
cellular nuclear antigen; or to enhance the induction of apoptosis by classical
chemotherapeutic drugs, such as placlitaxel in cell
lines MCF7 and MDAMB231, supporting their potential use as anti-cancer
therapies. These quantitative and explanatory results of the molecular
mechanisms could be inferred from the results of our research.
Marca et al. (23), who used the same
method as in this study, evaluated the in vitro genotoxicity of
ethanolic extract and Allium sativum L. (garlic) at concentrations of 5,
10, 50, 100, 200, 300, 400 and 500 mg/mL, found no genotoxic effect against
genomic DNA of Staphylococcus sp, while with the
garlic bulb juice, at concentrations of 5, 10, 50 and 100%, did show a powerful
genotoxic effect, fragmenting 100% of the genomic DNA of Staphylococcus
sp. They concluded that the juice of the bulb of Allium sativum L.
presents a powerful genotoxic activity against the genomic DNA of Staphylococcus
sp. In this sense, the turmeric has also presented genotoxic activity only with
the CE, starting at a concentration of 25 mg/mL, but it did not present
genotoxic activity with the EE, perhaps because the secondary metabolites
present in the CE responsible for the genotoxic activity are not found in the
EE, for not having been carried by the solvent or because they could minimally
change its chemical structure and lose its function. Meanwhile, curcumin, a chemically
pure compound, the main secondary metabolite of turmeric, did show moderate
genotoxic activity, but at a high concentration of 200 mg/mL.
Studies conducted on medicinal plants from other
families report that the genotoxic effect is attributed to the presence of
secondary metabolites, mainly phenolic compounds, tannins, and alkaloids.
Likewise, the metabolites present a synergistic effect, showing allelopathic
activity, causing damage to the cell and, particularly, to the DNA, leading to
cell death (30). However, the nuclease enzymes of Curcuma longa
L. have the property of fragmenting the DNA, and these enzymes could be present
in the extracts, exerting such a function. For this reason, to confirm that DNA
fragmentation is due to the action of secondary metabolites, in the Tomasevich method used, a tube was prepared with 100 mg/mL
of the extract plus the proteinase K enzyme and the DNA, and it was incubated
at 37 °C. If after the electrophoresis it is observed that the DNA has
been fragmented, it is by the action of the secondary metabolites and not by
the nuclease enzymes, since these enzymes of protein constitution would be
degraded by the action of the proteinase K enzyme during the incubation period.
In our study, the two treatments of the crude rhizome extract of Curcuma
longa L. at 100 mg/mL with and without proteinase K reveal similar behavior,
so it can be concluded that the fragmentation of DNA is due to the effect of
the secondary metabolites present in the extract.
It should be mentioned that there were limitations
related to economic financing to get the cellular lines DU‑145, HT-29, 3T3
BALB/c, which was finally provided by professionals and institutions that
collaborated in the research. Even though materials and reagents had to be
optimized, the results were not affected.
In conclusion, the Curcuma longa L. CE and EE show
different in vitro cytotoxic effects for the human tumor cell line
DU-145 and HT-29 similar to the standard compound curcumin. The Curcuma
longa L. CE presents a genotoxic effect according to its concentration,
while the EE does not. These results will allow the scientific community to
carry out studies to develop a phytomedicine that guarantees its therapeutic
use.
Acknowledgements
To Dr. Abraham J. Vaisberg Wolach, main professor of the Department of Microbiology
and researcher of the Cellular Biology and Virology Unit of the Universidad
Peruana Cayetano Heredia, for his valuable collaboration.
REFERENCES
1. Ministerio de Salud. Situación epidemiológica del cáncer de acuerdo a la vigilancia epidemiológica de cáncer basada en registros hospitalarios. Enero-diciembre 2017. Boletín epidemiológico del Perú Volumen 27 - SE 31. [Internet]. 2017 [citado 19 jun 2018]. Disponible en: http://www.dge.gob.pe/portal/docs/vigilancia/boletines/2018/05.pdf .
2. Siegel R, Miller K, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387.
3. Sciarra A, Gentilucci A, Salciccia S, Pierella F, Del Bianco F, Gentile V, et al. Prognostic value of inflammation in prostate cancer progression and response to therapeutic: a critical review. J Inflamm (Lond). 2016;13:35. doi: 10.1186/s12950-016-0143-2.
4. Morgillo F, Dallio M, DellaCorte C, Gravina A, Viscardi G, Loguercio C, et al. Carcinogenesis as a Result of Multiple Inflammatory and Oxidative Hits: a Comprehensive Review from Tumor Microenvironment to Gut Microbiota. Neoplasia. 2018;20(7):721-33. doi: 10.1016/j.neo.2018.05.002.
5. Garcia B, Saldaña A, Saldaña L. El estrés oxidativo y los antioxidantes en la prevención del cáncer. Rev Haban Cienc Méd. 2013;12(2):187-196. Disponible en: http://scielo.sld.cu/scielo.php?pid=S1729-519X2013000200005&script=sci_arttext&tlng=pt .
6. Aune D, Giovannucci E, Boffetta P, Fadnes L, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and allcause mortality–a systematic review and doseresponse meta-analysis of prospective studies. Int J Epidemiol. 2017; 46(3): 1029-56. doi: 10.1093/ije/dyw319.
7. Kapinova A, Kubatka P, Golubnitschaja O, Kello M, Zubor P, Solar P. Dietary phytochemicals in breast cancer research: anticancer effects and potential utility for effective chemoprevention. Environ Health Prev Med. 2018;23(1):36. doi: 10.1186/s12199-018-0724-1.
8. He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked?. Molecules. 2015;20(5):9183-9213. doi: 10.3390/molecules20059183.
9. Li M, Gar-Lee G, Kwok-Wing S, Kwok-Pui F, Bik-San C. Turmeric extract, with absorbable curcumin, has potent anti-metastatic effect in vitro and in vivo. Phytomedicine. 2018;(46):131-141. doi: 10.1016/j.phymed.2018.03.065.
10. González-Albadalejo J, Sanz D, Claramunt R, Lavandera J, Alkorta I, Elguero J. Curcumina y curcuminoides: química, estudios estructurales y propiedades biológicas. An R Acad Nac Farm. 2015;81(4):278-310. Disponible en: https://analesranf.com/wp-content/uploads/2015/81_04/8104_02.pdf.
11. Cosquillo M, Placencia M, Retuerto-Figueroa M, Gorriti A, Huamaní J. Caracterización físico-química y capacidad antioxidante de extractos del rizoma de Curcuma longa L. Rev Per Med Int. 2018;3(4):160-6. doi: 10.26722/rpmi.2018.34.97.
12. Vamanu E, Gatea F, Sârbu L, Pelinescu D. An In Vitro Study of the Influence of Curcuma longa Extracts on the Microbiota Modulation Process, In Patients with Hypertension. Pharmaceutics. 2019;11(4):191. doi: 10.3390/pharmaceutics11040191.
13. Mazidi M, Karimi E, Meydani M, Ghayour-Mobarhan M, Ferns G. Potential effects of curcumin on peroxisome proliferator activated receptor -γ in vitro and in vivo. World J Methodol. 2016; 6(1):112-117. doi: 10.5662/wjm.v6.i1.112.
14. Araki A, Quispe V, Pereira da Silva V, Rangel S, Estadella D, Barros M, et al. Putative mechanisms of genotoxicity induced by fluoride: a comprehensive review. Environ Sci Pollut Res. 2017; 24:15254–59. doi: 10.1007/s11356-017-9105-3.
15. Guerreiro N, Dinis-Oliveira R. Drugs of abuse from a different toxicological perspective: an updated review of cocaine genotoxicity. Arch Toxicol. 2018;92(10):2987-3006. doi: 10.1007/s00204-018-2281-1.
16. Silva de Sá I, Paula A, Leimann F, Vitoeia F, Bressan G, Krum, Nunes B, et al. In vitro and in vivo evaluation of enzymatic and antioxidant activity, cytotoxicity and genotoxicity of curcumin-loaded solid dispersions. Food Chem Toxicol. 2019; 125: 29-37. doi: 10.1016/j.fct.2018.12.037.
17. Verma R, Awasthi K, Rajawat N, Soni I, John P. Curcumin modulates oxidative stress and genotoxicity induced by a type II fluorinated pyrethroid, beta-cyfluthrin. Food Chem Toxicol. 2016; 97: 168-176. doi: 10.1016/j.fct.2016.09.014.
18. Skejan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J Natl Cancer Inst. 1990;82(13):1107-12. doi: 10.1093/jnci/82.13.1107.
19. Miranda T. Método Tomasevich: para determinar el efecto genotóxico in vitro de plantas medicinales y/o productos fitoterapéuticos. En: 2° Congreso Latinoamericano de Farmacogenómica y Medicina Personalizada: 25 al 28 de octubre del 2017. Durando: Dgo., México; 2017.
20. Gajskia G, Žegurab B, Ladeirac K, Novakb M, Sramkovae M, Pourrutf B, et al. The comet assay in animal models: From bugs to whales – (Part 2 Vertebrates). Mutat Res. 2019;781:130-164. doi: 10.1016/j.mrrev.2019.04.002.
21. Collins AR. The Comet Assay for DNA Damage and Repair. Mol Biotech. 2004;(26):249-261. doi: 10.1385/MB:26:3:249.
22. Moreno M, Miranda T, Quispe C, Rivera J, Ango H. Evaluación de la genotoxicidad in vitro de látex fresco y cristalizado de Carica papaya L. «papaya» frente a ADN genómico humano. En: 2° Congreso Latinoamericano de Farmacogenómica y Medicina Personalizada. 25 al 28 de octubre de 2017. Durando, Dgo., México; 2017.
23. Marca P, Miranda T, Moreno M, Galindo I, Arenas J. Preliminary evaluation of the in vitro genotoxicity of the ethanolic extract and allium sativum l. «garlic» juice against the DNA of Staphylococcus sp. En: 5to Congreso Internacional sobre Farmacología de Productos Naturales. 25 al 30 de mayo de 2018. Topes de Collantes, Cuba; 2018.
24. Miranda T, Moreno M, Alarcón G, Aguilar E, Infante G. Genotoxicidad in vitro de la planta medicinal Xanthium catharticum HBK «amor seco» frente a DNA genómico humano. En: 5to Congreso Internacional sobre Farmacología de Productos Naturales. Principios farmacológicos y evidencias científicas en la Medicina Tradicional y Natural. 25 al 30 de mayo de 2018. Topes de Collantes, Cuba; 2018.
25. Coico León AY, Juárez Pimentel AEY, Laurente Sánchez DI, Mantari Ochante FE, Alvarado Novoa AC, Gonzales Palomino M. Actividad citotóxica del extracto etanólico de Alternanthera mexicana en las líneas celulares 3T3 Y HUTU 80. Rev Per Med Int. 2016;1(2):5-11. Disponible en: http://www.ojs.rpmi.pe/index.php/RPMI/article/viewFile/12/16.
26. Kuete V, Seo E, Krusche B, Oswald M, Wiench B, Schröder S, et al. Cytotoxicity and Pharmacogenomics of Medicinal Plants from Traditional Korean Medicine. Evid Based Complement Alternat Med. 2013;2013:341724. doi: 10.1155/2013/341724.
27. Yue G, Jiang L, Kwok H, Lee J, Chan K, Fung K, et al. Turmeric ethanolic extract possesses stronger inhibitory activities on colon tumour growth than curcumin – The importance of turmerones. J Functional Foods. 2016; 22:565-77. doi: 10.1016/j.jff.2016.02.011.
28. Cao A, Li Q, Yin P, Dong Y, Shi H, Wang L, et al. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis. 2013;18(11):1391–1402. doi: 10.1007/s10495-013-0871-1.
29. Hong J, Ahn K, Bae E, Jeon S, Choi H. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006; 9(2):147-52. doi: 10.1038/sj.pcan.4500856.
30. Calaf G, Ponce-Cusi R, Carrión F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol Rep. 2018;40:2381-88. doi: 10.3892/or.2018.6603.
Correspondence to: Martha Francisca Cosquillo Rafael; Av. La Cantuta 294, Zárate, San Juan de Lurigancho, Lima, Perú;
martha.cosquillo@unmsm.edu.pe.
Contributions of the authors: MFCR, MDPM, TYMT, MMH and MGRF designed the article,
collected the data, carried out the statistical analysis, wrote the manuscript
and approved the final version.
Conflicts of Interest: The authors declare no conflict of interest with the publication of
this article.
Funding:
Self-funded.
Cite as: Cosquillo-Rafael MF, Placencia-Medina
MD, Miranda-Tomasevich TY, Moreno-Hinojosa M, Retuerto-Figueroa MG. In vitro cytotoxic and genotoxic effect of the crude and ethanolic extract from the rhizome of Curcuma longa L. Rev Peru Med Exp Salud Publica.
2020;37(3)454-61. doi: https://doi.org/10.17843/rpmesp.2020.373.4817.
The article is part of the thesis of Cosquillo-Rafael M.: “Efecto antioxidante, antitumoral y genotóxico del extracto crudo y etanólico del rizoma de Curcuma longa L. “palillo” [Master’s Thesis]. Lima: Facultad de Medicina, Universidad Nacional Mayor de San Marcos; 2019.