María Teresa Álvarez-Bañuelos
Jaime Morales-Romero
Christian S. Ortiz-Chacha
Clara Luz Sampieri-Ramírez
10.17843/rpmesp.2020.373.4929
ORIGINAL ARTICLE
Place of residence and social marginalization asprognostic factors for prostate cancer survival in Veracruz, Mexico
Richy
Rogelio Gutiérrez-Juárez
![]() , Medical Doctor
, Medical Doctor
María Teresa Álvarez-Bañuelos
![]() , Doctor of Biological Sciences
, Doctor of Biological Sciences
Jaime Morales-Romero
![]() , Doctor of Public Health Sciences
, Doctor of Public Health Sciences
Christian S. Ortiz-Chacha
![]() , Doctor of Health Sciences
, Doctor of Health Sciences
Clara Luz Sampieri-Ramírez
![]() , Doctor of Biological Sciences
, Doctor of Biological Sciences
ABSTRACT
Objectives: To determine if the place of residence and the level of poverty are associated with prostate cancer survival.
Materials and methods: All patients diagnosed with prostate cancer (PC) in the period from 2013 to 2017 in a tertiary healthcare hospital in Veracruz, Mexico were included. Patients resided in rural and urban areas. Variables were collected according to clinical-epidemiological and histopathological characteristics. The Kaplan Meier method and the Log Rank test were used to measure survival. Prognostic factors were determined by calculating the adjusted hazard ratio (HRa) in a multivariate analysis using the Cox proportional risk method.
Results: A total of 186 PC cases were analyzed. Overall, after 5 years, 48.3% of the patients survived. Men living in urban areas had a higher probability of survival than those living in rural areas (HRa 1.67, 95% CI 1.16-2.41). Similarly, people living in areas classified as low-poverty zones had a higher probability of survival than those living in areas with high-poverty (HRa 2.32, 95% CI 1.47-3.66).
Conclusions: To reside in a rural place was identified as a negative prognostic factor for the survival of patients with PC regardless of other sociodemographic and clinical variables; patients living in places with a high-poverty level had an unfavorable survival prognosis.
Keywords: Prostatic Neoplasms; Survival Analysis; Rural Population;Socioeconomic Factors; Mexico (Source: MeSH NLM).
INTRODUCTION
Internationally, prostate cancer (PC) is the secondmost prevalent cancer in men (33.1 cases per 100,000 people) after lung cancerand is the fifth leading cause of death from malignant neoplasms in the malepopulation (1). In Mexico, the number of deaths from malignantprostate tumors has increased in the last two decades (2). PC iscaused by several factors, such as age, diet, ethnicity, skin color, exposureto tobacco or alcohol, and even some infections (3,4).
In Mexico, the PC panorama is characterized by agrowing incidence, a greater frequency of advanced stages, increased managementcosts and an increase in mortality (5,6).It has been documented that PC survival is related to the clinical stage at themoment of diagnosis and the type of treatment used (5). In thissense, there is evidence of 5-year survival in different types of cancer, suchas lung, liver, colorectal, breast, pancreatic, esophageal, bladder, andprostate (7). In addition, some studies have shown longersurvival of patients with PC who lived in urban areas and with a highersocioeconomic level, compared to those who came from rural areas and with a lowsocioeconomic level (8,9).
The association of such characteristics with survivalhas been barely studied in Mexican population, and the information about Veracruzpopulation is almost nonexistent. The aim of this study was to determine if theplace of residence and the marginalization level are associated to prostatecancer survival in a hospital cohort.
| KEY MESSAGES |
|
Motivation for the study: No recent studies have been found to evaluate prostate cancer survival in Mexico, so the factors associated are unknown. Main findings: Low prostate cancer survival rates were obtained, in addition, it was observed that living in rural areas and a high marginalization level are poor prognosis factors for survival. Implications: More studies are necessary to identify the sociodemographic factors and implications associated with this public health problem, it is also required to strengthen health-related actions aimed at generating interventions that increase survival to prostate cancer. |
MATERIALS AND METHODS
Study design and participants
A retrospective open cohort study was carried out inwhich all patients diagnosed with PC in the period between 2013-2017 wereincluded from the State Cancer Center (CECan), whichis a third-level hospital located in Xalapa,Veracruz, Mexico. The follow-up period was 60 months from the registration ofthe first PC case identified during the recruitment period. The applied inclusioncriteria were patients diagnosed with PC within the study period and whoreceived care at that hospital; the exclusion criteria were patients with ahistory of other types of cancer or who received previous antineoplastictreatment; and the elimination criteria were patients diagnosed with PC withoutcontact information for follow-up.
Variables
The main response variable was the survival time ofpatients with PC defined as the time, in months, between the date of PC diagnosis and the patient's death. Death was verified by the death certificate provided by the hospital's social work coordination and through the records ofthe Epidemiological and Statistical System of Deaths of the national healthsystem.
The sociodemographic covariables were schooling (withor without studies), marginalization level of the place of residence (veryhigh, high, medium, low or very low) according to the Consejo Nacional de Población, which takes into account eightsocioeconomic indicators (percentage of illiterate population 15 years orolder, without complete primary education, homes without tap water services,electricity, refrigerator, or toilet, with dirt floor and average number ofinhabitants per room) (10); the area of habitual residence in ruralareas, if it was a place with less than 2,500 inhabitants, or in urban areas,if they habitually lived in places with more than 2,500 inhabitants, accordingto the classification of the Instituto Nacional de Estadística y Geografía (INEGI) (11); and occupation (agricultural and related activities vs. other activities).
The clinical-histopathological variables werecomorbidity (presence or absence of diabetes mellitus or arterialhypertension), prostate specific antigen (PSA); <10 ng/dL; 10 to 20 ng/dL;>20 ng/dL, histologic differentiation (well-differentiated,moderately-differentiated, and poorly-differentiated), Gleason Scale score (≤6,7, or ≥8), metastasis (present or absent), clinical stage (I, II, III, or IV,according to the criteria of the American Joint Committee on Cancer, AJCC). Dependingon tumor extension, there are two stages: early stage (localized tumor), and advancedstage (tumor extends through the prostatic capsule or invades adjacentstructures), and two types of treatment: neoadjuvant hormone therapy (beforeprostatectomy) and adjuvant (after prostatectomy) (12).
Specific survival was calculated considering the periodbetween the date of the histopathological diagnosis and the date of death orthe end of the follow-up, whichever occurred first.
Data collection instrument
A data collection card was designed and validated bythree specialized doctors, both from the hospital where the study was carriedout and from the National Institute of Oncology and Radiobiology of Havana,Cuba. The information source was the patients´ clinical file. The date andcause of death were obtained from the death certificate or from the records ofthe Epidemiological and Statistical System of Deceases (SEED).
Statistical analysis
The Fisher's chi-square or exact test on categorical variables was used to compare the demographic and clinical characteristics between rural and urban strata, and the Student's t-test was used to compareage groups. Survival analysis was calculated with the Kaplan-Meier method; patients who were still alive at the end of follow-up or whose current status was unknown were considered as censored data. Survival probabilities for each possible prognostic factor were compared by means of the Log Rank test, initially specifying a crude model for each variable to be analyzed. Â Subsequently, proportional risk models were adjusted by Cox regression. Adjustment variables were included in the model, according to the background review on the most important prognostic variables related to prostate cancer survival (9,13,14). During the multivariate analysis, the covariables were dichotomized, includingthe total sample: age at the time of diagnosis, place of habitual residence, social marginalization level, clinical stage, histological differentiation grade andtype of treatment. Raw and adjusted hazard ratios (HR) and their respective 95% confidence intervals were calculated. The analyses were carried out through thestatistical software SPSS 23.0 (IBM Inc., NY, USA).
Ethical aspects
The research protocol was submitted and approved bythe Technical Council of the Public Health Institute of the Universidad Veracruzana and by the Committee of Research Ethics of the National Center of Cancerology "Dr. Miguel Dorantes Mesa" , with registration number C.E.I.-2018-044.
RESULTS
General characteristics
We identified 198 patients with PC, 12 of whom did not meet the inclusion criteria and were excluded. Finally, the files of 186 patients were analyzed. The lethality was 28.5% (n = 53).Follow-up was not completed in 8.6% (n = 16) of the cases. The mean age at diagnosis was 69.2 ± 8.96 years, and the mean age at death was 76.3.
Differences between urban and rural groups
Regarding the place of residence, 62.4% of the patients lived in rural areas. Differences were statistically significant when comparing the proportions of the rural and urban groups by schooling, marginalization level, occupation, and presence of metastasis (Table 1).
Table 1. Sociodemographic andclinical-pathological characteristics of the cohort according to the place ofresidence.
|
Variable |
Place of residence |
p value |
|
|
Urban |
Rural |
||
|
n = 70 (%) |
n = 116 (%) |
||
|
Age |
|
|
|
|
Mean (SD) |
68 (±8.4) |
70 (±9.2) |
0.100 b |
|
Schooling |
|
|
|
|
Without studies |
19 (27.1) |
52 (44.8) |
0.016 a |
|
With studies |
51 (72.9) |
64 (55.2) |
|
|
Socio-economic marginalization level |
|
|
|
|
Very low - low |
25 (35.7) |
18 (20.5) |
0.002 a |
|
Medium |
31 (44.3) |
53 (60.2) |
|
|
High - very high |
14 (20.0) |
17 (19.3) |
|
|
Occupation |
|
|
|
|
Agricultural and related |
47 (67.1) |
104 (89.7) |
<0.00 a |
|
Other |
23 (32.9) |
12 (10.3) |
|
|
Comorbidity (diabetes or hypertension) |
|
|
|
|
Yes |
16 (22.9) |
34 (29.3) |
0.336 a |
|
No |
54 (77.1) |
82 (70.7) |
|
|
Gleason Scale Score |
|
|
|
|
≤6 |
29 (41.4) |
60 (51.7) |
|
|
7 |
19 (27.1) |
16 (13.8) |
0.073 a |
|
≥8 |
22 (31.4) |
40 (34.5) |
|
|
Clinical stage |
|
|
|
|
I |
3 (4.3) |
7 (6.0) |
0.057 c |
|
II |
19 (27.1) |
30 (25.9) |
|
|
III |
36 (51.4) |
40 (34.5) |
|
|
IV |
12 (17.1) |
39 (33.6) |
|
|
Histologic grade |
|
|
|
|
Well differentiated |
4447 (10.0) |
8 (6.9) |
|
|
Moderately differentiated |
27 (38.6) |
44 (32.9) |
0.726 a |
|
Poorly differentiated |
36 (51.4) |
64 (55.2) |
|
|
Presence of metastasis |
|
|
|
|
Positive |
12 (17.1) |
39 (33.6) |
0.023 a |
|
Negative |
58 (82.9) |
77 (66.4) |
|
|
Location of metastasis |
|
|
|
|
Bone |
8 (75.0) |
28 (71.8) |
0.054 a |
|
Other |
4 (25.0) |
11 (28.2) |
|
a Chi-square test, b Student's t-test, c Fisher exact test
When comparing the clinical pathological characteristics, stage III was the most frequent in both areas. In contrast, stage IV was found 3.25 times less in patients with habitual urban residence compared to those in rural areas. In the same way, bone metastases were 3 times more frequent in those of urban zone than from rural zone (Table 1).
Survival analysis
Overall survival for subjects in the 2013 cohort was70.6% (95% CI: 62.1 to 77.5) at 1 year; 70.6% (95% CI: 62.1 to 77.5) at 3years, and 47.7% (95% CI: 31.7 to 63.0) at 5 years.
Statistically significant differences were observed insurvival by level of marginalization, where patients with high/very high marginalization level presented lower survival than those coming from areas ofvery low or low marginalization. Likewise, the survival of patients coming fromareas of medium marginalization was even greater than those of low/exceptionally low marginalization (Table 2).
Table 2. Cohort subjects´ survival at 1, 3and 5 years of follow-up.
|
Variable |
Patients alive |
Survival a |
p value b |
||
|
1 year |
3 years |
5 years |
|||
|
n (%) |
n (%) |
n (%) |
|||
|
Age (years) |
|
|
|
|
|
|
≤60 |
35 |
27 (76) |
16 (60) |
4 (25) |
0.051 |
|
>60 |
151 |
131 (87) |
96 (73) |
54 (56) |
|
|
Place of residence |
|
|
|
|
|
|
Rural |
116 |
93 (80) |
56 (60) |
54 (28) |
<0.001 |
|
Urban |
70 |
64 (91) |
49 (77) |
35 (71) |
|
|
Schooling |
|
|
|
|
|
|
Without studies |
71 |
58 (81) |
35 (61) |
16 (45) |
0.118 |
|
With studies |
115 |
100 (87) |
76 (76) |
37 (49) |
|
|
Occupation |
|
|
|
|
|
|
Agricultural and related |
151 |
124 (82) |
88 (71) |
45 (51) |
0.289 |
|
Other |
35 |
33 (94) |
22 (68) |
7 (32) |
|
|
Socio-economic marginalization level |
|
|
|
|
|
|
Very low - low |
43 |
38 (89) |
26 (69) |
18 (69) |
<0.001 |
|
Medium |
84 |
80 (95) |
73 (91) |
59 (81) |
|
|
High - very high |
59 |
41 (69) |
20 (48) |
3 (18) |
|
|
Clinical stage |
|
|
|
|
|
|
I |
10 |
10 (100) |
10 (100) |
9 (86) |
<0.001 |
|
II |
49 |
46 (94) |
39 (85) |
30 (77) |
|
|
III |
76 |
67 (88) |
47 (71) |
20 (43) |
|
|
IV |
51 |
34 (66) |
15 (46) |
4 (29) |
|
|
Gleason Scale |
|
|
|
|
|
|
Low risk |
15 |
15 (100) |
14 (92) |
10 (76) |
0.152 |
|
Moderate risk |
75 |
63 (84) |
47 (74) |
31 (67) |
|
|
High risk |
96 |
79 (82) |
50 (64) |
7 (13) |
|
|
Metastasis |
|
|
|
|
|
|
Present |
51 |
34 (66) |
15 (46) |
4 (29) |
<0.001 |
|
Absent |
135 |
123 (91) |
97 (79) |
52 (54) |
|
|
Metastasis location |
|
|
|
|
|
|
Bone |
40 |
27 (68) |
12 (44) |
3 (28) |
0.955 |
|
Other |
11 |
6 (58) |
4 (58) |
1 (29) |
|
|
Type of treatment |
|
|
|
|
|
|
Adjuvant |
93 |
83 (89) |
54 (77) |
28 (44) |
0.031 |
|
Neoadjuvant |
93 |
73 (79) |
46 (63) |
21 (46) |
|
a Kaplan-Meiermethod, b Log Rank test
Depending on the clinical stage, lower survival was observed in stage IV subjects compared to stages I and II. According to the degree of histological differentiation (Figure 1), the cumulative proportion that survived at the end of the 60-month interval was 73% in the well-differentiated group, 70% in the moderately differentiated group, and 30% in the poorly differentiated group (p = 0.035).
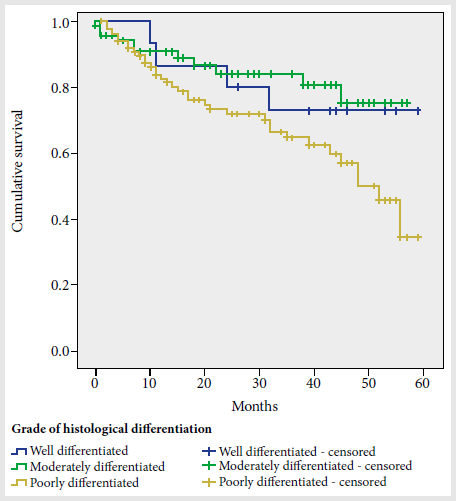
Figure 1. Prostate cancer cumulative survival curve according to histological differentiation grade.
Lower survival was observed among subjects with serumprostate-specific antigen (PSA) levels greater than 20 ng/dL prior totreatment, compared to those with levels of 10-20 and <10 ng/dL (Figure 2); the 5-year survival probabilities were 31%, 83%, and 84%, respectively (p =0.003).
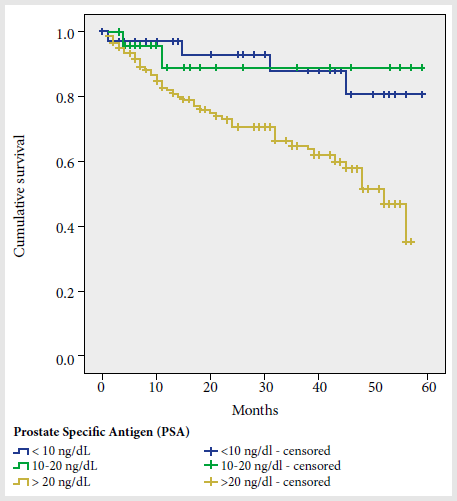
Figure 2. Prostate cancer cumulative survival curve according to prostate antigen levels.
Table 3. Predictors of survival in prostate cancer patients
|
Variable |
No adjusted model |
p value |
Multivariate model |
p value |
|
HR (95% CI) |
HR (95% CI) |
|||
|
Usual place of residence |
|
|
|
|
|
Rural |
1.00 |
0.013 |
1.00 |
0.006 |
|
Urban |
1.56 (1.10-2.22) |
1.67 (1.16-2.41) |
||
|
Socioeconomic marginalization level |
|
|
|
|
|
High |
1.00 |
<0.001 |
1.00 |
<0.001 |
|
Low |
2.29 (1.46-3.60) |
2.32 (1.47-3.66) |
||
|
Clinical stage |
|
|
|
|
|
Advanced |
1.00 |
0.359 |
1.00 |
0.443 |
|
Early |
1.18 (0.83-1.68) |
1.15 (0.80-1.66) |
||
|
Age at diagnosis (years) |
|
|
|
|
|
>60 |
1.00 |
0.306 |
1.00 |
0.942 |
|
≤60 |
1.28 (0.80-2.05) |
1.02 (0.63-1.66) |
||
|
Histological differentiation grade |
|
|
|
|
|
Barely differentiated |
1.00 |
0.427 |
1.00 |
0168 |
|
Clearly differentiated |
1.15 (0.82-1.62) |
1.29 (0.90-1.84) |
||
|
Treatment type |
|
|
|
|
|
Adjuvant |
1.00 |
0.909 |
1.00 |
0.956 |
|
Neoadjuvant |
0.98 (0.70-1.39) |
1.01 (0.71-1.43) |
HR: Hazard ratio; 95% CI: 95% confidence interval.
A multivariate analysis of possible survival predictors in prostate cancer patients is presented in Table 3. In the adjusted model, the characteristics of habitual residence, such as urban type (HRa: 1.67, 95% CI: 1.16 to 2.41) and a low marginalization level (HRa: 2.32, 95% CI: 1.47 to 3.66) showed a greater probability of survival than those coming from a rural type locality or with a high marginalization level. No significant differences were found in the probability of survival with respect to clinical stage, age at diagnosis, histological differentiation, and type of treatment.
DISCUSSION
The results found show a mean age at the time of diagnosis of 69 years similar to those reported in other previous studies conducted in Mexico (15), Brazil (16), and Colombia (17),in which the mean age and standard deviation were 66.7 (±8.8), 70.5 (±8.7) and69 (±8.6) years, respectively. Fatality was at 28%, similar to the 24% reported by Silveira et al. in 2013 in a Brazilian hospital (18).
Nearly 68% of the cases were diagnosed during advanced stages (stage III and IV), a figure higher than the one reported in another study carried out in the Mexican population (15), in which only 46.2% of cases were reported in stages III and IV. Differences could be explained by the fact that each of the hospitals where the research took place out serves a population with different socio-demographic characteristics, mainly regarding their usual place of residence, occupation and schooling.
It is worth mentioning that in some countries, such asthe United States and New Zealand, people living in rural or less advantaged areas have a lower probability of survival, less access to health services, higher mortality, lower rates of PSA testing, and a higher risk of advanced PC (8).This is why, it is necessary to state that geographic location provides information related to population composition and access to certain resources and services, among other aspects; while the degree of advantage reflects the probable influence of the community and social structure on the risk of developing the disease, regardless of individual socioeconomic level (8). Regarding survival according to place of habitual residence, some studies have shown alower survival rate in people of rural residence with prostate cancer (7,14,19).
It is essential to highlight that, among the findingsof this study, a specific survival to PC at 5 years was observed near 48%, a figure equivalent to almost half of what was reported by other Latin American countries included in the largest study carried out internationally (CONCORD-3) (20).In this study, figures above 80% are reported in some cases such as Brazil,Costa Rica, Argentina, Ecuador and Uruguay; while in the Colombian population according to Arias and De Vries (21) this probability is around 70%(95% CI: 65.3 to 76.1).
It should be clarified that, the mentioned studies analyzed population-based data, unlike this one in which hospital-based datawere collected. Also, the patients included in this study had the same insurance regime (Seguro Popular), came in greater proportion from rural areas (62.4%) and required to move from their place of residence to the CECan, located in the capital of the State of Veracruz. Then,these patients faced mainly geographical and economical barriers, as well aslong waiting times for diagnosis and treatment, which may have limited or delayed their access to medical care (22,23) and, consequently, their probability of survival decreased.
In contrast, when the probability of survival at 1, 3,and 5 years was compared (84.6%, 70.6%, 47.7%, respectively) to other hospital-based studies, this study´s findings were similar to those found in another research conducted in Iran, in which figures of 87%, 73%, and 54%, respectively, were reported (24).
In relation to 5-year survival in stage I patients (86%), our findings were similar to those reported by Migowsky et al. (16) in a study conducted in Brazil with a sample of 258 patients, in which the survival was of 87.8% (95% CI: 83.3 to 92.5).
Results obtained from this study provide arguments to claim that in a high proportion of advanced stage patients (68.3%), survival decreased as the stage increased. Reported survival was of 29% in stage IV, almosthalf of what was found in other studies (between 40% and 60%) (17,18,24). These data show that the case identification or the search for attention are late, decreasing the probability of survival, which suggests the need to increase efforts for detection in early stages (18).
As for survival by place of habitual residence, some studies have shown a lower survival rate in persons of rural residence with PC (7,8,19). Li et al. (7) found that5-year survival to different types of cancer, including lung, liver, colorectal, breast, pancreatic, esophageal, bladder, and prostate was higher in patients from urban areas compared to those from rural areas (44.05 vs. 41.47%,p < 0.001); while, with respect to PC specifically, it was also higher inurban areas with a statistically significant difference (59.2% vs. 53.3%, p =0.02).
There is evidence of variations in survival rates between different geographic areas, demonstrated mainly in studies comparing rural and urban cancer patients. Such variations may be related to exposure torisk factors, as well as to differential conditions for access to health services and therefore to early detection tests and timely treatment of cancer (7,25,26).
In this regard, a meta-analysis conducted in 2015 by Baade et al (8), which included works from different high-income countries, showed that the survival risk ratio was almost 1.7 times higher in patients living in urban areas compared to those in rural areas.
Differences found in the marginalization level were also found in this study. In the adjusted model, patients with a low marginalization level showed greater survival compared to those with a high marginalization level. Similar results in previous studies demonstrated an association betweena high socioeconomic level and a greater probability of survival as can be seenin the analysis of Bravo et al (27), where men diagnosed with PC who lived in more economically advantageous areas had better survival than those living in less advantageous areas, independently of the clinical stage atthe time of diagnosis (HR: 3.5, 95% CI 2.37 to 5.40).
The analyzed studies confirm the association between the degree of deprivation or socioeconomic disadvantage and survival to PC in other countries. In all of them, it has been observed that survival is lower inmen with greater socioeconomic deprivation compared to those with higher or more advantageous socioeconomic levels (14,21,28-30).
In the case of Mexico, in the states with higher marginalization, there are fewer technological and human resources for the care of health problems. However, economic, geographic, educational and cultural factors, could be related to the reduction in made diagnosis and timely treatment, which may influence PC survival in people living in places with such characteristics and which support the results found in thisstudy (23).
The main limitations for this study include its retrospective nature and the restrictions inherent to the information quality from the clinical files. From these files, a small proportion of the patients' clinical stage at the time of diagnosis was identified; thus, by determining the stagefrom the other clinical data, errors in classification could have been generated. In addition, the level of marginalization of each case was stablished by the catalogs prepared at a certain time by geographical area, sothis data may not reflect the individual reality of the patients.
In conclusion, the place of rural residence was identified as a poor prognostic factor for the survival of patients with PC, regardless of other sociodemographic and clinical variables. Likewise, patients living in places with higher marginalization levels had an unfavorable survival prognosis. Differences in survival due to sociodemographic characteristics show the need to reduce the inequality gaps, especially for people living in rural areas and with higher marginalization level.
REFERENCES
1. IARC/OMS. Global observatory of cancer [Internet]. GLOBOCAN 2018. 2018 [citado el 10 de mayo de 2018]. Disponible en: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
2. Instituto Nacional de Estadística y Geografía. Principales causas de mortalidad por residencia habitual, grupos de edad y sexo del fallecido. [Internet]. 2017 [citado el 2 de diciembre de 2017]. Disponible en: http://www.inegi.org.mx/est/contenidos/proyectos/registros/vitales/mortalidad/tabulados/ConsultaMortalidad.asp.
3. Ferrís-Tortajada J, García-Castell J, Berbel -Tornero O, Ortega- García JA. Constitutional risk factors in prostate cancer. Actas Urol Esp. 2011;35(5):282–8. doi: 10.1016/j.acuroe.2011.06.005.
4. Bashir MN. Epidemiology of prostate cancer. Asian Pac J Cancer Prev. 2015;16(13):5137–41. doi: 10.1016/j.acuroe.2011.06.005.
5. Torres-Sánchez LE, Espinoza-Giacinto R, Rojas-Martínez R, Escamilla-Nuñez C, Vázquez-Salas RA, Campuzano JC, et al. Prostate cancer mortality according to marginalization status in Mexican states from 1980 to 2013. Salud Publica Mex. 2016;58(2):179–86. doi: 10.21149/spm.v58i2.7787.
6. Mohar-Betancourt A, Reynoso-Noveron N, Armas-Texta D, Gutierrez-Delgado C, Torres-Dominguez JA. Cancer trends in Mexico : Essential data for the creation and follow-up of public policies. Am Soc Clin Oncol. 2017;3(6):740–8. doi: 10.1200/JGO.2016.007476.
7. Li X, Deng Y, Tang W, Sun Q, Chen Y, Yang C. Urban-Rural disparity in cancer incidence, mortality, and survivals in Shanghai, China, during 2002 and 2015. Fontiers Oncol. 2018;8(3):71–80. doi: 10.3389/fonc.2018.00579.
8. Baade PD, Yu XQ, Smith DP, Dunn J, Chambers SK. Geographic disparities in prostate cancer outcomes - Review of international patterns. Asian Pacific J Cancer Prev. 2015;16(3):1259–75. doi: 10.7314/APJCP.2015.16.3.1259.
9. Rapiti E, Fioretta G, Schaffar R, Neyroud-caspar I. Impact of Socioeconomic Status on Prostate Cancer Diagnosis , Treatment , and Prognosis. Cancer. 2009;115(23):5556–65. doi: 10.1002/cncr.24607.
10. Consejo Nacional de Población. Metodología de estimación del índice de marginación por localidad [Internet]. 2010. Disponible en: http://www.conapo.gob.mx/es/CONAPO/Indice_de_Marginacion_por_Localidad_2010. Published 2010.
11. Teresa M, Macgregor G De. Desarrollo y distribución de la población urbana en México. Investig Geogr. 2003;(50):77–91.
12. Secretaria de Salud. Diagnóstico y Tratamiento del Cáncer de próstata en Segundo y Tercer nivel de Atención. Guía Práctica Clínica GPC [Internet]. 2009;1–102. Disponible en: www.cenetec.salud.gob.mx.
13. Thomas AA, Pearce A, Sharp L, Gardiner RA, Chambers S, Aitken J, et al. Socioeconomic disadvantage but not remoteness affects short-term survival in prostate cancer: A population-based study using competing risks. Asia Pac J Clin Oncol. 2017;13(2):e31–40. doi: 10.1111/ajco.12570.
14. Yu XQ, Luo Q, Smith DP, O’Connell DL, Baade PD. Geographic variation in prostate cancer survival in New South Wales. Med J Aust. 2014;200(10):586–90. doi: 10.5694/mja13.11134.
15. Cayetano-Alcaraz AA, Ramírez-Rivera JA, Sotomayor-de-Zavaleta M, Castillejos-Molina RA, Gabilondo-Navarro F, Feria-Bernal G, et al. Características de los casos incidentes de cáncer de próstata en los últimos 5 años en un hospital de tercer nivel en México. Rev Mex Urol. 2016;76(2):76–80. doi: 10.1016/j.uromx.2015.11.007.
16. Migowski A, Silva GA. Survival of patients with clinically localized prostate cancer. Rev Saude Publica. 2010;44(2):344–52. doi: 10.1590/s0034-89102010000200016.
17. Villegas C, Chacón J, Sánchez T. Sobrevida en cáncer de próstata de una población del centro de Colombia. Acta Med Colomb. 2015;40(2):101–8. doi: 10.36104/amc.2015.447.
18. Silveira F, Bandeira Lages R, Assuncao Costa U, Mendes Teles J, Campelo V. Survival of patients with prostate cancer. Rev Bras Promoc Saude. 2013;26(1):45–50.
19. Afshar N, English DR, Milne RL. Rural–urban residence and cancer survival in high-income countries: A systematic review. Cancer. 2019;135(3):2172–84. doi: 10.1002/cncr.32073.
20. Allemani C, Matsuda T, Carlo V Di, Harewood R, Matz M, Nikšić M, et al. Articles Global surveillance of trends in cancer survival 2000 – 14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;14(391):1023–75. doi: 10.1016/S0140-6736(17):33326-3.
21. Arias-Ortiz NE, de Vries E. Inequidades en salud y supervivencia al cáncer en Manizales, Colombia : un estudio de base poblacional. Colomb Med. 2018;49(6):63–72. doi: 10.25100/cm.v49i1.3629.
22. Hernández Morales M, Hernández Nieves A, García Grajales S, Ocampo Escobedo R, Luna Ruiz M, Ortiz Chacha C, et al. Mortalidad y factores sociales del cáncer de próstata para la gestión de políticas públicas. Veracruz, México. Investig Andin. 2017;19(35):163–83. doi: 10.33132/01248146.956.
23. Torres-Sánchez LE, Espinoza-Giacinto R, Rojas-Martínez R, Escamilla-Nuñez C, Vázquez-Salas RA, Campuzano JC, et al. Prostate cancer mortality according to marginalization status in Mexican states from 1980 to 2013. Salud Publica Mex. 2016;58(2):179–86.
24. Moghimi M, Shayestehpour M. Survival outcome in men with prostate cancer in Yazd Province, Central Iran, from 2001 to 2012. Asian Pacific J Cancer Biol. 2017;2(4):79–82. doi: 10.22034/APJCB.2017.2.4.79.
25. Hashibe M, Kirchhoff AC, Kepka D, Kim J, Millar M, Sweeney C, et al. Disparities in cancer survival and incidence by metropolitan versus rural residence in Utah. Cancer Med. 2018;7(4):1490–7. doi: 10.1002/cam4.1382.
26. Dasgupta P, Baade PD, Aitken JF, Ralph N, Chambers SK, Dunn J. Geographical variations in prostate cancer outcomes: A systematic review of international evidence. Front Oncol. 2019;9(2):238–73. doi: 10.3389/fonc.2019.00238.
27. Cortés A, Bravo LE, García LS, Collazos P. Incidencia, mortalidad y supervivencia por cáncer de próstata en Cali, Colombia, 1962-2012. Salud Publica Mex. 2014;56(5):457–64. doi: 10.21149/spm.v56i5.7371.
28. Eylert M, Bahl A, Hounsome L, Verne J, Jefferies E, Persad R. The impact of socio-economic deprivation on incidence, treatment and mortality from prostate cancer in England, 1990–2010. J Clin Urol. 2016;9(2):93–101. doi: 10.1177/2051415815594976.
29. Tomic K, Ventimiglia E, Robinson D, Häggström C, Lambe M, Stattin P. Socioeconomic status and diagnosis, treatment, and mortality in men with prostate cancer. Nationwide population-based study. Int J Cancer. 2018;142(12):2478–84. doi: 10.1002/ijc.31272.
30. Klein J, von dem Knesebeck O. Socioeconomic inequalities in prostate cancer survival: A review of the evidence and explanatory factors. Soc Sci Med. 2015;142:9–18. doi: 10.1016/j.socscimed.2015.07.006.
Correspondence to: María Teresa Ãlvarez-Bañuelos; talvarez@uv.mx
Authors' contribution: GJRR participated in the study design; data collection, analysis and interpretation; and manuscript writing. ABMT participated in the conception, design, data analysis and interpretation, and manuscript writing and revision. MRJ, OCCS, and SRCL participated in the data analysis and interpretation, and manuscript revision. All authors assume responsibility for each of the sections, and declare that they have reviewed the content and approved the final version of the article.
Conflict of Interest: Authors declare no conflict of interest
Cite as: Gutiérrez-Juárez RR, Álvarez-Bañuelos MT, Morales-Romero J, Ortiz-Chacha C, Sampieri CL. Place of residence and social marginalization as prognostic factors for prostate cancer survival in Veracruz, Mexico. Rev Peru Med Exp Salud Publica. 2020;37(3). doi: https://doi.org/10.17843/rpmesp.2020.373.4929
Funding: Self-funded.