Salyoc Tapia-Rojas
Alejandro Fukusaki-Yoshizawa
Álvaro Marcelo-Rodríguez
José Amiel-Pérez
10.17843/rpmesp.2020.373.5157
ORIGINAL ARTICLE
Cytotoxic activity of the chloroform fraction of Piper aduncum and its effect on the cell cycle in gastric cancer cell lines
Ana Mayanga-Herrera
![]() , Genetic biologist and biotechnologist,
Master of Science in Biotechnology
, Genetic biologist and biotechnologist,
Master of Science in Biotechnology
Salyoc Tapia-Rojas
![]() , Bachelor in Genetics
and Biotechnology
, Bachelor in Genetics
and Biotechnology
Alejandro Fukusaki-Yoshizawa
![]() , Chemist, Magister
Scientiae
, Chemist, Magister
Scientiae
Álvaro Marcelo-Rodríguez
![]() , Biologist, Doctor of Biochemistry
, Biologist, Doctor of Biochemistry
José Amiel-Pérez
![]() , Pharmaceutical Chemist, Doctor of Pharmacy and Biochemistry
, Pharmaceutical Chemist, Doctor of Pharmacy and Biochemistry
ABSTRACT
Objectives: To evaluate the cytotoxic activity of the chloroform fraction of the Piper aduncum methanolic extract (PAMoCl) and its effect on the cell cycle in two gastric cancer cell lines: AGS and KATO III.
Materials and methods: The cytotoxic effect of PAMoCl was evaluated in cell lines AGS and KATO III. The following PAMoCl concentrations were tested, 1.25, 2.5, 5, 10, 20, 40, 80 and 160 μg/mL. Resazurine was used to evaluate cell viability. In the cell cycle assay, the cells were treated with 19.62 μg/mL and 39.23 μg/mL of PAMoCl for AGS as well as 87.49 μg/mL and 160 μg/mL for KATO III. After 24 hours both cell lines were analyzed by flow cytometry.
Results: PAMoCl showed cytotoxic activity, inhibiting cell growth by 50%. It presented a (IC50) of 39.23 μg/mL and 87.49 μg/mL at 24 hours and a (IC50) of 49.47 μg/mL and 64.68 μg/mL at 48 hours against AGS and KATO III cell lines, respectively. In addition, it was observed that PAMoCl has an effect on the cell cycle, it causes an accumulation of cells in the G2/M phase.
Conclusions: PAMoCl contains secondary metabolites with cytotoxic activity that have an effect on the G2/M phase of the cell cycle, in two gastric cancer cell lines, both primary and metastatic. The results of this study will allow us to deepen the search for more effective active ingredients found in PAMoCl for eliminating gastric cancer cells, but with less toxicity for healthy cells.
Keywords: Gastric Cancer, Cytotoxicity, Cell Cycle, Chloroform, Metastasis (Source: MeSH NLM).
INTRODUCTION
Gastric cancer is the third most frequent cause of cancer death worldwide (1) and the leading cause in Peru (2). It is usually detected in advanced stages where effective treatment is almost impossible. Since chemotherapy side effects are highly toxic, it has become urgent to seek new drugs with greater specificity against cancer cells, greater overall effectiveness and fewer side effects.
Medicinal
plants are being studied as a source of new chemotherapeutic products. Nowadays,
about 60% of the drugs used to treat cancer are derived from plants, for
example, paclitaxel, initially obtained from Taxus
brevifolia Nutt. (3); camptotecin, from Captotheca
acuminata (4); etoposide, from Podophyllum species (5); vincristine,
from Catharanthus roseus (6);
and colchicine, from Colchicum autumnale (7).
The Piper genus consists of 700 species found in different places across the world. Most of these species have several positive effects on health, such as gastrointestinal and liver protection (8). In addition, many species of the Piper genus have shown cytotoxic activity against cell lines from breast, prostate, ovarian, pancreas, liver, colon and cervical cancer among others (9).
It has been reported in previous studies that
the ethanolic extract from the Piper aduncum species presents cytotoxic activity against
MCF-7 (breast cancer) and NCI-H460 (lung carcinoma) cells (10). However, the effect
of the chloroform fraction from the Piper aduncum
methanolic extract (PAMoCl)
on gastric cancer cells has not been reported yet. Therefore, the aim of this
study was to evaluate the cytotoxic activity of PAMoCl
and its effect on the cell cycle in two gastric cancer cell lines: AGS and KATO
III.
|
KEY MESSAGES |
|
Motivation for the study: Due to the high mortality rate of gastric cancer in Peru, it is important to know if metabolites with cytotoxic activity against this type of cancer can be found in our environment. Main
findings: The chloroform fraction of Piper aduncum
has cytotoxic activity, which causes the cell cycle to stop in the G2/M phase
in 2 gastric cancer cell lines, one of them being the metastatic cell line. Implications: Since the
chloroform fraction has cytotoxicity against a metastatic gastric cancer cell
line, identification of the metabolites responsible for this activity would
be necessary for new treatments against metastatic gastric cancer. |
MATERIALS AND METHODS
Preparation of the chloroform fraction from Piper aduncum
Piper
aduncum leaves were collected from the lower Kimiri area, district of La Merced, province of Chanchamayo, department of Junin,
at coordinates 11°02'16.5 "S 75°18'54.0 "W. Leaves were collected in
the wild, and one sample was taxonomically identified as Piper aduncum L. by the natural history museum of the
Universidad Nacional Mayor de San Marcos. The preparation of the Piper aduncum methanolic extract (PAMo) and the Piper aduncum
chloroform fraction (PAMoCI) was completed under standardized
protocols at the laboratory of Chemistry and Biochemistry of Natural Products
of the Universidad Científica del Sur (11).
The
leaves were cleaned and dried at 40 °C during two days, then crushed and
sieved with a 1 mm mesh. Then 70 g of powdered leaves were weighed,
300 mL of methanol was added and filtered using a Whatman
No. 1 filter paper. This procedure was repeated eight times, from which
the last three were sonicated for 2 hours. The entire PAMo
was filtered using a 0.44 µm membrane. To obtain the PAMoCI,
the PAMo was concentrated to 200 mL and extracted
by adding 300 mL of chloroform in a 1 L decanting tube. This process
was repeated 8 times. The PAMoCl was concentrated at
reduced pressure with a rotary evaporator. The PAMo
and the PAMoCl were analyzed by thin-layer
chromatography (TLC) using silica gel 60 for the stationary phase and
benzene-acetone 8:1 as the mobile phase; and it was then developed with
iodine and ultraviolet (UV) light (12). Complementary tests were
conducted to identify chemical groups for both PAMo
and PAMoCl according to protocols described by Lock (13).
Finally, the PAMoCl was treated to a concentration of
32 mg/mL in dimethyl sulfoxide (DMSO) and stored at –80 °C for later
use. The analytical solvents were purchased from Merck.
Cell line culture
The
human gastric cancer cell lines AGS (primary) and KATO III (metastatic) were
acquired from the European Authenticated Cell Culture Collection (ECACC
89090402 and 86093004), and cell line 293T (ATCC® CRL-3216TM) from the
laboratory of Molecular Genetics of the Universidad Cientifica
del Sur.
DMEM-F12
medium (Biowest), supplemented with 10% fetal bovine
serum and 1% antibiotic-antifungal solution (full DMEM-F12), was used to
culture AGS and 293T cells. RPMI medium (Biowest)
supplemented with 20% fetal bovine serum and 1% antibiotic-antifungal solution
(Biowest) was used to culture the KATO III cell line.
All cells were incubated at 37 °C with 5% CO2 and subcultured when confluence was 70-80%.
Cellular viability assay
The
cell viability assay was performed as previously described (14). AGS
and 293T cells were counted using a Neubauer chamber
and 5 × 103 cells/well were seeded in 96-well plates. KATO
III cells were seeded in a quantity of 104 cells/well. All cells
were incubated for 12 hours, then treated with 1.25 µg/mL, 2.5 µg/mL,
5 µg/mL, 10 µg/mL, 20 µg/mL, 40 µg/mL, 80 µg/mL, and 160 µg/mL
of PAMoCl, and incubated again for 24 and 48 hours. In
addition, a control group with the vehicle (DMSO at 0.5%), not the PAMoCl, was included. For cell viability assay, 20 µL
of resazurine (0.15 mg/mL) was added to each
well and incubated for 3 hours. Finally, the 96-well plates were read with a
Synergy LX (Biotek) multimodal plate reader by spectrophotometry
at wavelengths of 570 nm and 600 nm. The PAMoCl
concentration, that causes 50% cell growth inhibition (GI50) with
respect to the control group growth, was calculated.
Cellular morphology observation
Changes
in cell morphology after incubation of AGS, KATO III and 293T cells with PAMoCl were observed and photographed on an inverted phase-contrast
microscope (Nikon Eclipse TI) after 24 and 48 hours.
Cellular cycle evaluation by flow cytometry
The Darzynkiewicz et al. protocol (15), with
slight modifications, was used to evaluate the effect of PAMoCl
on the cell cycle. A total of 350,000 AGS and 500,000 KATO III cells were
seeded in 100 × 15 mm petri dishes with DMEM-F12 and RPMI media.
After 24 hours, the culture medium was replaced with a new DMEM-F12 medium and a
complete RPMI containing PAMoCl at concentrations of
19.62 and 39.23 µg/mL for AGS cells and 87.49 and 160 µg/mL for KATO
III cells; these concentrations were obtained from the cell viability assay.
All plates were incubated for 24 hours at 37 °C and 5% CO2. After
24 hours, the plates were washed twice with phosphate buffer saline (PBS) 1X and
trypsinized for 5 minutes. The resuspended
cells were collected by centrifugation and fixed with cold 70% ethanol; then
they were incubated at 4 °C for 30 minutes. Afterwards, the cells
were stained with a propidium iodide solution (50 µg/mL)
and RNase (100 µg/mL) for an additional 30 minutes and immediately
analyzed in the Guava EasyCyte flow cytometer
(Merck).
Data analysis
The
absorbance data obtained from the cell viability assay were exported to a
Microsoft Excel file and expressed as percentages with respect to the control
group. For the dose-response relationship and the calculation of GI50,
a non-linear regression model was used. The significant differences between
groups were compared using the one-way ANOVA test with the Tukey test, as a post
hoc test (p < 0.05), using the GraphPad Prism
program. The cell cycle results were analyzed using the FCS 7 Express program (DeNovo solutions). The analyzed data are the result of
three independent experiments.
RESULTS
The
presence of metabolites in PAMo and PAMoCl was evidenced by TLC (Figure 1). The identification
of the chemical groups are detailed in Table 1.
PAMoCl: chloroform
fraction of Piper aduncum methanolic
extract;
PAMo: Piper aduncum methanolic extract
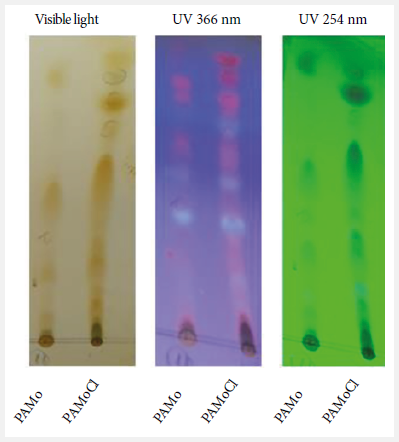
Figure 1. Thin layer chromatography
profile of Piper aduncum methanolic
extract and chloroform fraction of Piper aduncum
methanolic extract revealed with iodine (visible
light) and UV light. Mobile phase: benzene-acetone 8:1. Stationary phase:
silica gel 60.
Table 1. Metabolite groups
present in Piper aduncum methanolic
extract and its chloroform fraction
|
Metabolite group |
Test |
PAMo |
PAMoCl |
|
Phenolics |
FeCl3 |
Positive |
Positive |
|
Flavonoids |
Shinoda |
Positive |
Positive |
|
Anthocyanins |
Rosenhein |
Positive |
Positive |
|
Triterpenoids and steroids |
Lieberman-Buchard |
Positive |
Positive |
PAMoCl: chloroform
fraction of Piper aduncum methanolic
extract
PAMo: methanolic extract of Piper aduncum
Effect of the chloroform fraction of the Piper aduncum methanolic extract on
cell viability
In
the cell viability assay, all evaluated cell lines showed cytotoxic activity of
PAMoCl with the following GI50 results at
24 hours: 39.23 µg/mL, 87.49 µg/mL and 74.10 µg/mL; and at 48
hours 49.47 µg/mL; 64.68 µg/mL and 101.8 µg/mL for AGS, KATO III
and 293T cell lines, respectively (Figure 2). At 48 hours, PAMoCl
GI50 was significantly lower for AGS and KATO III lines compared to
293T (cytotoxicity control) with values of p < 0.01 and p < 0.05,
respectively (Figure 3).
PAMoCl: Chloroform fraction of Piper aduncum methanolic extract
GI50: 50% cell
growth inhibition
R2: Coefficient
of determination of the non-linear regression model
Figure 2. Effect of the chloroform fraction of Piper aduncum
methanolic extract (PAMoCl)
on cell viability. Cell lines 293T, AGS and KATO III were treated at different
concentrations of PAMoCl (1.25; 2.5; 5; 10; 20; 40;
80 and 160 µg/mL) during 24 and 48 hours. GI50 graphs are
shown, which are log10 dependent cell viability curves (%) of PAMoCl concentration (µg/mL) for each cell line. In the
upper right part of each graph, GI50 values are shown at 24 and 48
hours.
a p < 0.05; b p < 0.01; ns: not significant
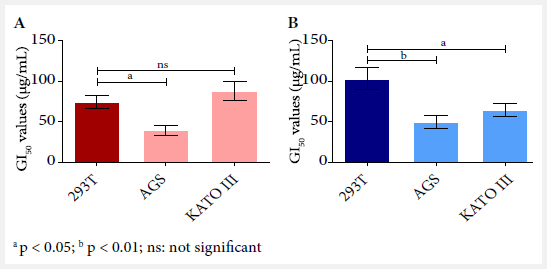
Figure 3. Comparison of 50% cell growth inhibition (GI50) values for
each cell line at (A) 24 hours and (B) 48 hours
Observation of cell morphology
PAMoCl caused dose-dependent changes in the morphology of AGS, KATO III and
293T cells (Figure 4). After a 24-hour treatment with PAMoCl,
starting at 20 µg/mL, AGS cells began to contract and others resuspended. At higher concentrations, such as 80 µg/mL
and 160 µg/mL, more cell fragments, few resuspended
cells and none attached were observed. After 48 hours the effect was
similar. In the KATO III cell line, dead cells (Figure 4) are observed starting
from 80 µg/mL, at both 24 and 48 hours. After a 24-hour treatment
with PAMoCl, 293T cells began to contract at 80 µg/mL. At 160 µg/mL more contracted and resuspended cells were observed, however, cells could still
be found adhering to the culture plate. At 48 hours, the observed effect was
similar.
Figure 4. Effect of the chloroform fraction of Piper aduncum methanolic extract at
5 µg/mL, 20 µg/mL and 80 µg/mL concentrations on the morphology
of gastric cancer cell lines AGS, KATO III and human kidney 293T. A control
group of cells without treatment but with the vehicle (DMSO 0.5%) is also shown
for each cell line. T.M. 100X. Red arrow: resuspended
cells; blue arrow: attached cells; white arrow: contracted cells; and black
arrow: dead cells.
Effect on the cell cycle
Results
showed an effect on the cell cycle from the gastric cancer lines. The
percentage of AGS cells in G2/M phase was 31.8%; 44.1% and 52.7% for the
concentrations of 0 µg/mL; 19.62 µg/mL and 39.23 µg/mL of PAMoCl, respectively (Figure 5 A-C). The percentage of KATO III
cells in G2/M phase was 30.9%; 29.3% and 49.0% for the concentrations of 0 µg/mL;
87.49 µg/mL and 160 µg/mL of PAMoCl,
respectively (Figure 5 D-F).
PAMoCl: Chloroform fraction of Piper aduncum methanolic extract
Figure
5. Flow cytometry analysis for the cell cycle of
gastric cancer cell lines AGS and KATO III that were treated with the
chloroform fraction of Piper aduncum methanolic extract (PAMoCl).
Histograms are shown indicating the amount of DNA marked by propidium
iodide. Three groups are considered for each cell line: A) control group with
AGS cells without PAMoCl, but with the vehicle; B)
AGS cells treated with 19.62 μg/mL of PAMoCl; C) AGS cells treated with 39.23 μg/mL of PAMoCl; D) control group
with KATO III cells without treatment but with the vehicle; E) KATO III cells
treated with 87.49 μg/mL of PAMoCl;
F) KATO III cells treated with 160 μg/mL of PAMoCl. Raw data from flow cytometry (black curve), G1: Gap
1 (red curve), S: synthesis (blue curve), and G2/M: Gap 2/mitosis (green
curve), cell cycle phases.
DISCUSSION
This
is the first reported study in which the cytotoxic activity of PAMoCl and its effect on the cell cycle is demonstrated in
two gastric cancer cell lines: AGS and KATO III.
The GI50 values in this study for
both cell lines are lower than those reported by Herrera et al. for the ethanolic extract of P. aduncum
in the MCF-7, HT-29, K-562 and H-460 cell lines (10). It is
important to mention that the GI50 values found for the AGS and KATO
III lines are significantly lower than those of the 293T line, which is an
embryonic human kidney cell line and was used as toxicity control. This shows
that PAMoCl has a greater effect on gastric cancer
cells than on noncancerous cells.
Various
metabolites found in plants from the Piper genus have shown biological
activity, especially alkaloids. For example, piperine
has been shown to inhibit tumor growth and metastasis of lung cancer cells (16).
Pipernonalin shows activity against human prostate
cancer cells (17). Amides may also inhibit growth in cancer cell
lines (18).
In
this study, it was observed that PAMoCl has a
concentration-dependent effect on the cell cycle, which causes the treated
cells to stop in the G2/M phase. This effect was observed in both AGS and KATO
III cells. These results coincide with those reported for other plant metabolites
that have shown the same effect in G2/M phase. For example, roscovitin
(purines) inhibited the expression of proteins p53, CDK7, cyclin A, cyclin E
and CDK2 in non-small cell lung cancer (19), lymphoma (20)
and breast cancer (21). Sulforaphane (isothiacyanates) increased the expression of cyclin B1 and
p21 (22). Quercetin (flavonols) in
hepatocellular carcinoma cells overexpressed p53, p21 and decreased the
expression of cyclin D1, CDK2 and CDK7 (23). Finally, berberine (alkaloids) inhibited the expression of cyclin B1
and increased the expression of Wee1 in leukemia cells (24).
Similar
to what was found in this study, several species from Piper genus have
metabolites with effects on the G2/M phase. For example, piperin
from P. nigrum and P. longum
in osteosarcoma cells (25), flavkawaina
from P. methysticum (26), hinokinine from P. cubeba (27),
hydroxychavicol from P. betle
(28); and piperlongumina from P. longum L. showed decreased expression of cyclin D1 in
AGS cells (29).
In
the phytochemical analysis, 4 metabolite groups were detected in PAMoCl: phenolic groups and derivatives, flavonoids,
anthocyanins, triterpenoids and steroids. This is why we consider important to study
the identification of secondary metabolites using analytical techniques, such
as chromatography, infrared spectroscopy, mass spectrometry and nuclear
magnetic resonance that could allow us to elucidate the chemical structure of
the active compounds.
However,
it should be noted that there are limitations to this study. For example, the
time and place for sample collection, the method of extraction, among others,
factors that can generate variability in the quantity of metabolites and,
consequently, in their biological activity.
In
conclusion, it is reported that PAMoCl contains
secondary metabolites with cytotoxic activity that causes the cell cycle to
stop in the G2/M phase in two gastric cancer cell lines both primary and
metastatic. The results from this study will allow to further search for active
principles present in PAMoCl that have greater
efficacy in eliminating gastric cancer cells, but with less toxicity for
healthy cells.
Acknowledgements:
To
Dr. Juan Manuel Iglesias, head of the Molecular Genetics Laboratory of the Universidad
Científica del Sur, for the
donation of the 293T cell line used in this study.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
2. Torres-Román JS, Grados-Sánchez O. Cáncer gástrico en el Perú: una realidad susceptibilidad de cambio. Rev Gastroenterol del Perú [Internet]. 2015;35(3):276. Disponible en: http://www.revistagastroperu.com/index.php/rgp/article/view/118/115 .
3. Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235(1–2):179–92. doi: 10.1016/s0378-5173(01)00986-3.
4. Thomas CJ, Rahier NJ, Hecht SM. Camptothecin: current perspectives. Bioorg Med Chem. 2004;12(7):1585–604. doi: 10.1016/j.bmc.2003.11.036.
5. Meresse P, Dechaux E, Monneret C, Bertounesque E. Etoposide: discovery and medicinal chemistry. Curr Med Chem. 2004;11(18):2443–66. doi: 10.2174/0929867043364531.
6. Moore A, Pinkerton R. Vincristine: Can its therapeutic index be enhanced? Pediatr Blood Cancer. 2009;53(7):1180–7. doi: 10.1002/pbc.22161.
7. Lin Z-Y, Kuo C-H, Wu D-C, Chuang W-L. Anticancer effects of clinically acceptable colchicine concentrations on human gastric cancer cell lines. Kaohsiung J Med Sci. 2016;32(2):68–73. doi: 10.1016/j.kjms.2015.12.006.
8. Kumar N, Misra P, Dube A, Bhattacharya S, Dikshit M, Ranade S. Piper betle Linn. a maligned Pan-Asiatic plant with an array of pharmacological activities and prospects for drug discovery. Curr Sci. 2010;99(7):922–32. Disponible en: https://www.jstor.org/stable/24066069 .
9. Wang YH, Morris-Natschke S, Yang J, Niu HM, Long CL, Lee KH. Anticancer principles from medicinal Piper (Hú Jiāo) plants. J Tradit Complement Med. 2014;4(1):8–16. doi: 10.4103/2225-4110.124811.
10. Herrera-Calderon O, Alvarado-Puray C, Arroyo-Acevedo J, Rojas-Armas J, Chumpitaz-Cerrate V, Hañari-Quispe R, et al. Phytochemical screening, total phenolic content, antioxidant, and cytotoxic activity of five peruvian plants on human tumor cell lines. Pharmacognosy Res. 2018;10(2);161-165. doi: 10.4103/pr.pr_109_17.
11. Amiel-Pérez J, Fukusaki A, Enciso N, Altamirano C, Herrera AM, Marcelo Á, et al. Interferencia de pigmentos vegetales al aplicar la técnica XTT a extractos de Buddleja globosa, Senecio tephrosiodes Turcz. Y Equisetum giganteum. Científica. 2016;13(1):9-26. doi: 10.21142/cient.v13i1.318.
12. Bladt S. Plant Drug Analysis: A thin layer chromatography atlas. Springer Science & Business Media; 2009. doi: 10.1007/978-3-642-00574-9.
13. Lock OR. Invetigacion Fitoquimica, Metodos en el estudio de productos naturales. 1ra. edición. Lima: Pontificia Univ Catolica Peru; 2016.
14. Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, et al. Cell Viability Assays. Assay Guid Man [Internet]. 2004;(Md):1–25. Disponible en: http://www.ncbi.nlm.nih.gov/pubmed/23805433 .
15. Crowley LC, Chojnowski G, Waterhouse NJ. Measuring the DNA content of cells in apoptosis and at different cell-cycle stages by propidium iodide staining and flow cytometry. Cold Spring Harb Protoc. 2016;2016(10):pdb-prot087247. doi: 10.1101/pdb.prot087247.
16. Lai L, Fu Q, Liu Y, Jiang K, Guo Q, Chen Q, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin. 2012;523. doi: 10.1038/aps.2011.209.
17. Lee W, Kim K-Y, Yu S-N, Kim S-H, Chun S-S, Ji J-H, et al. Pipernonaline from Piper longum Linn. induces ROS-mediated apoptosis in human prostate cancer PC-3 cells. Biochem Biophys Res Commun. 2013;430(1):406–12. doi: 10.1016/j.bbrc.2012.11.030.
18. Bezerra DP, Pessoa C, de Moraes MO, Silveira ER, Lima MAS, Elmiro FJM, et al. Antiproliferative effects of two amides, piperine and piplartine, from Piper species. Zeitschrift für Naturforsch C. 2005;60(7–8):539–43. doi: 10.1515/znc-2005-7-805.
19. Zhang F, Zhang T, Gu Z-P, Zhou Y-A, Han Y, Li X-F, et al. Enhancement of radiosensitivity by roscovitine pretreatment in human non-small cell lung cancer A549 cells. J Radiat Res. 2008;49(5):541–8. doi: 10.1269/jrr.08024.
20. Lacrima K, Rinaldi A, Vignati S, Martin V, Tibiletti MG, Gaidano G, et al. Cyclin-dependent kinase inhibitor seliciclib shows in vitro activity in diffuse large B-cell lymphomas. Leuk Lymphoma. 2007;48(1):158–67. doi: 10.1080/10428190601026562.
21. Węsierska‐Gądek J, Gritsch D, Zulehner N, Komina O, Maurer M. Interference with ER‐α enhances the therapeutic efficacy of the selective CDK inhibitor roscovitine towards ER‐positive breast cancer cells. J Cell Biochem. 2011;112(4):1103–17. doi: 10.1002/jcb.23024.
22. Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L, et al. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer. 2004;48(2):198–206. doi: 10.1207/s15327914nc4802_10.
23. Li Y, Duan S, Jia H, Bai C, Zhang L, Wang Z. Flavonoids from tartary buckwheat induce G2/M cell cycle arrest and apoptosis in human hepatoma HepG2 cells. Acta Biochim Biophys Sin. 2014;46(6):460–70. doi: 10.1093/abbs/gmu023.
24. Khan M, Giessrigl B, Vonach C, Madlener S, Prinz S, Herbaceck I, et al. Berberine and a Berberis lycium extract inactivate Cdc25A and induce α-tubulin acetylation that correlate with HL-60 cell cycle inhibition and apoptosis. Mutat Res Mol Mech Mutagen. 2010;683(1–2):123–30. doi: 10.1016/j.mrfmmm.2009.11.001.
25. Zhang J, Zhu X, Li H, Li B, Sun L, Xie T, et al. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int Immunopharmacol. 2015;24(1):50–8. doi: 10.1016/j.intimp.2014.11.012.
26. Ji T, Lin C, Krill LS, Eskander R, Guo Y, Zi X, et al. Flavokawain B, a kava chalcone, inhibits growth of human osteosarcoma cells through G2/M cell cycle arrest and apoptosis. Mol Cancer. 2013;12(1):55. doi: 10.1186/1476-4598-12-55.
27. Cunha NL, Teixeira GM, Martins TD, Souza AR, Oliveira PF, Símaro GV, et al. (−)-Hinokinin Induces G2/M Arrest and Contributes to the Antiproliferative Effects of Doxorubicin in Breast Cancer Cells. Planta Med. 2016;82(06):530–8. doi: 10.1055/s-0042-101761.
28. Guha Majumdar A, Subramanian M. Hydroxychavicol from Piper betle induces apoptosis, cell cycle arrest, and inhibits epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Pharmacol. 2019;166:274-291. doi: 10.1016/j.bcp.2019.05.025.
29. Song B, Zhan H, Bian Q, Gu J. Piperlongumine inhibits gastric cancer cells via suppression of the JAK1, 2/STAT3 signaling pathway. Mol Med Rep. 2016;13(5):4475–80. doi: 10.3892/mmr.2016.5091.
Correspondence to: Ana Mayanga-Herrera;
amayanga@cientifica.edu.pe
Authors’
contributions: AMH, STR and JAP participated in the conception and design of the
article, data collection and critical review. AFY participated in the
elaboration of the methanolic extract and the
chloroform fraction of P. aduncum used in this
study. AMH and STR conducted the experiments, analyzed and interpreted data,
and elaborated the discussion. AMR assisted in the results interpretation. All
authors participated in the writing and approval of the final version of the
article.
Conflicts
of interest: The authors declare no conflict of interest.
Funding
Sources: This work was co-funded by the Consejo
Nacional de Ciencia, Tecnología
e Innovación Tecnológica
(CONCYTEC) and the Universidad Científica del Sur,
via the "Evaluation of the effect of extracts and fractions of three
medicinal plants on gastric cancer stem cells" project, contract No.
134-2017-FONDECYT.
Cite as: Mayanga-Herrera A,
Tapia-Rojas S, Fukusaki-Yoshizawa A,
Marcelo-Rodríguez A, Amiel-Pérez J. Cytotoxic
activity of the chloroform fraction of Piper aduncum
and its effect on the cell cycle in gastric cancer cell lines. Rev Peru Med Exp Salud Publica.
2020;37(3). doi:
https://doi.org/10.17843/rpmesp.2020.373.5157.