Jim Brian Valenzuela-Medina
Eduardo Gotuzzo
Fernando Alonso Mejía-Cordero
Elsa Violeta González-Lagos
Brief report
Cancer in people living with HIV-AIDS at a referral hospital in Lima, Peru
Lucía Maryelena Mendoza-Mori ![]() 1, Physician
1, Physician
Jim Brian Valenzuela-Medina ![]() 1, Physician
1, Physician
Eduardo Gotuzzo ![]() 1, Infectious and Tropical Diseases Physician
1, Infectious and Tropical Diseases Physician
Fernando Alonso Mejía-Cordero ![]() 1, Infectious and Tropical Diseases Physician
1, Infectious and Tropical Diseases Physician
Elsa Violeta González-Lagos ![]() 1, Physician, Master in Clinical Epidemiology
1, Physician, Master in Clinical Epidemiology
1 Instituto de Medicina Tropical "Alexander von Humboldt", Universidad Peruana Cayetano Heredia, Lima, Perú.
ABSTRACT
This study was carried out to describe and compare the demographic, clinical, and therapeutic characteristics of HIV patients who developed some cancer. We identified 276 cancer cases diagnosed at Hospital Cayetano Heredia between 2000 and 2018. 80,8% (223/276) had AIDS-defining-cancers (ADCs), being Kaposi's Sarcoma the most frequent type; meanwhile, among non-AIDS-defining-cancers (NADCs), the most frequent was Hodgkin lymphoma. The median age was 36,5 years, being highest among the cases diagnosed with NADCs. Concerning CD4 lymphocyte counts, the median among ADCs was much lower than NADCs, 87,5 cells/µl and 216 cells/µl, respectively. Therefore, NADCs cases have a longer history of HIV infection, and an older age at cancer diagnosis, as well as higher CD4 cells counts.
Keywords: HIV, AIDS, Neoplasms, Latin America (source: MeSH NLM).
INTRODUCTION
The risk of developing cancer in people living with HIV (PLHIV) is higher than that of the general population; this is a consequence of the immunosuppression caused by this virus, which impairs the control of viral infections with oncogenic potential (1,2). After the introduction of antiretroviral therapy (ART) worldwide, the morbidity and mortality rate due to opportunistic infections and some cancers decreased considerably (3). In developed countries, where this therapy is widely available, PLHIV have a longer life expectancy and neoplasms are positioned as one of the main causes of death (4).
According to the Centers for Disease Control and Prevention (CDC) classification criteria (5), HIV-associated cancers are classified into AIDS-defining neoplasms (ADN) and non-AIDS-defining neoplasms (NADN). ADN include Kaposi’s sarcoma (KS), non-Hodgkin’s lymphoma (NHL) and invasive cervical cancer (ICC), all caused by oncogenic viruses such as human herpes virus type 8 (HHV-8), Epstein-Barr virus (EBV) and human papillomavirus (HPV), respectively (5). And NADN include all remaining types of cancers. Thus, since the widespread use of ART, the incidence of ADN has largely decreased, while the number of NADN has increased in recent years (6,7).
In Peru there is little evidence on neoplasms in PLHIV. Of the few articles, those on KS and non-Hodgkin’s lymphoma stand out. For example, Mohanna et al. found an incidence of KS of 20 per 1,000 patients treated for HIV between 1987 and 2003 (8). While, in 2018, Cuellar et al. carried out a study to determine prognostic factors for non-Hodgkin’s lymphoma in HIV patients. To date, there is not a study that describes the characteristics of HIV patients who developed any type of cancer (9). Therefore, much of the information comes from studies conducted in other countries, where the epidemiological characteristics and the context of the population are different from those in Peru (10-12). For this reason, the aim of our study was to describe and compare the demographic, clinical, and therapeutic characteristics of PLHIV who developed cancer in a public referral hospital in Peru.
|
KEY MESSAGES |
|
Motivation for the study: The risk of developing cancer in people living with HIV is higher than that of the general population. However, there are no studies carried out in Peru on this subject. Main findings: The most frequent non-AIDS-defining neoplasms were Kaposi’s sarcoma and non-Hodgkin’s lymphoma; whereas, among non-AIDS-defining neoplasms, Hodgkin’s lymphoma was the most common. Implications: These findings represent a first insight regarding cancer in patients with HIV/AIDS in Peru. |
THE STUDY
Design and study population
We conducted a cross-sectional and descriptive study, based on information obtained from the records of the HIV-AIDS cohort (COVIHS), which to date is enrolling about 9,000 PLHIV receiving specialized care in the National Health Strategy for the Prevention and Control of STIs, HIV and AIDS (ESNITSS) at the Cayetano Heredia National Hospital. All PLHIV over 18 years of age who developed any type of cancer between January 1, 2000 and December 31, 2018, and who were enrolled in that cohort were selected.
Variable definition
Demographic characteristics included age at cancer diagnosis, sex, and self-reported transmission method. Cancer-related variables included cancer type (ADN or NADN), calendar year of neoplasm diagnosis (period 2000-2004, 2005-2009, 2010-2014 or 2015-2018), and time from HIV diagnosis to cancer. Cancer diagnosis was taken into account up to one year prior to HIV diagnosis, and at any time after HIV diagnosis (13).
Laboratory values were CD4 cell count (<200 cells/μL or ≥200 cells/μL) and Log10 viral load (suppressed; 2.6-3.9; 4.0-4.9 or ≥5.0 copies/mL), both based on the most recent result registered between six months before and one month after the date of cancer diagnosis (13). Cancer treatment comprised chemotherapy, radiotherapy, surgery, or a combination thereof. Intraepithelial neoplasms (CIN) were not considered. Missing data were kept and classified in the unknown group.
Data Management
The information was obtained through authorized extraction from the COVIHS database. Personal data such as names and surnames were associated with a numerical code. Subsequently, a new database was created in Excel, respecting the same numerical identifiers. Only the researchers had access to the final database, and the main researchers were responsible for its processing and statistical analysis. To guarantee the quality of the information, we verified for inconsistencies, errors and duplicate data.
Statistical analysis
Descriptive analysis was summarized using median and interquartile range (IQR) for continuous variables, and percentages for categorical variables. To compare variables between the ADN and NADN groups, we applied the Chi-square test and the Mann-Whitney U test, as appropriate. A p value of less than 0.05 was considered statistically significant. Data harmonization and processing was carried out with the STATA 14 statistical package.
Ethical considerations
The study protocol was approved by the Institutional Ethics Committee of the Universidad Cayetano Heredia. Informed consent was not required because the data came from a database and was analyzed retrospectively.
FINDINGS
From 2000 to 2018, 276 cancer cases were identified in 269 PLHIV; 80.8% (223/276) had ADN and 19.2% (53/276) NADN. The most frequent ADN were Kaposi’s sarcoma with 62.8% (140/223) and non-Hodgkin’s lymphoma with 30.0% (67/223); while, regarding NADN, Hodgkin’s lymphoma with 22.6% (12/53) was the most frequent (Table 1).
Table 1. Frequency of cancer types diagnosed at Cayetano Heredia Hospital between 2000-2018 (n = 276).
|
Types of cancer |
n (%) |
|
Cancer-defining neoplasms (n = 223) |
|
|
Kaposi's sarcoma |
140 (62.8) |
|
Non-Hodgkin's lymphoma |
67 (30.0) |
|
Invasive cervical cancer |
16 (7.2) |
|
Non-cancer-defining neoplasms (n = 53) |
|
|
Hodgkin's lymphoma |
12 (22.6) |
|
Cervical cancer in situ |
9 (17.0) |
|
Skin cancer |
7 (13.2) |
|
Other locations * |
25 (47.2) |
*Includes stomach, lungs, anus, rectum, eyes, breast, endometrium, ovaries, liver, prostate, tongue.
The median age of the total number of cases was 36.5 years (IQR: 29.7-46.0); and 79.4% (219/276) were men. Men who have sex with men (MSM) were more numerous in the ADN group, whereas heterosexuals predominated among NADN. The median time from HIV diagnosis to cancer was 9.2 months (IQR: 1.4-52.7); however, 77.4% (41/53) of NADN had a time greater than 12 months from HIV diagnosis to cancer. The median CD4 cell count was 112 cells/µL (IQR: 39.5-253.0), with about 60% (160/276) having levels below 200 cells/µL, and only 25.4% (70/276) in viral suppression at cancer diagnosis (Table 2).
Table 2. Demographic, clinical and therapeutic characteristics of cases diagnosed with cancer at Cayetano Heredia Hospital between 2000-2018
|
Characteristics |
Total |
ADN |
NADN |
p value |
|
|
n (%) |
n (%) |
n (%) |
|||
|
Age |
|
||||
|
Median (IQR) |
36.5 (29.7-46.0) |
35.2 (29.4-43.7) |
42.2 (32.3-55.6) |
0.001 a |
|
|
Sex |
|
|
|
|
|
|
Male |
219 (79.3) |
187 (83.9) |
32 (60.4) |
<0.001 b |
|
|
Female |
57 (20.7) |
36 (16.1) |
21 (39.6) |
|
|
|
Transmission method |
|
|
|
|
|
|
Heterosexual |
133 (48.2) |
95 (42.6) |
38 (71.7) |
<0.001 b |
|
|
MSM |
140 (50.7) |
126 (56.5) |
14 (26.4) |
|
|
|
Unknown |
3 (1.1) |
2 (0.9) |
1 (1.9) |
|
|
|
Period in years |
|
|
|
|
|
|
2000-2004 |
13 (4.7) |
11 (4.9) |
2 (3.8) |
0.886 b |
|
|
2005-2009 |
53 (19.2) |
45 (20.2) |
8 (15.1) |
|
|
|
2010-2014 |
96 (34.8) |
76 (34.1) |
20 (37.7) |
|
|
|
2015-2018 |
114 (41.3) |
91 (40.8) |
23 (43.4) |
|
|
|
Time from HIV diagnosis to cancer |
|
|
|
|
|
|
Median (IQR) |
9.2 (1.4-52.7) |
5.8 (1.1-39.1) |
52.7 (16.9-99.3) |
<0.001 a |
|
|
Pre-cancer diagnoses |
9 (3.3) |
6 (2.7) |
3 (5.7) |
|
|
|
0-2 months |
75 (27.2) |
71 (31.8) |
4 (7.5) |
|
|
|
2-12 months |
62 (22.4) |
57 (25.6) |
5 (9.4) |
|
|
|
>12 months |
130 (47.1) |
89 (39.9) |
41 (77.4) |
|
|
|
CD4 cell count (cells/ÁL) |
|
|
|
|
|
|
Median (IQR) |
112 (39.5-253) |
87.5 (37-241) |
216 (129-298) |
<0.001 a |
|
|
< 200 |
160 (58.0) |
140 (62.8) |
20 (37.7) |
|
|
|
≥ 200 |
84 (30.4) |
58 (26.0) |
26 (49.1) |
|
|
|
Unknown |
32 (11.6) |
25 (11.2) |
7 (13.2) |
|
|
|
Viral load (copies/mL) |
|
|
|
|
|
|
Median (IQR) |
4.7 (2.4-5.4) |
4.9 (3.3-5.5) |
1.9 (1.6-3.5) |
<0 .001 a |
|
|
Suppressed |
70 (25.4) |
39 (17.5) |
31 (58.5) |
|
|
|
2.6-3.9 |
29 (10.5) |
22 (9.9) |
7 (13.2) |
|
|
|
4.0-4.9 |
46 (16.7) |
41 (18.4) |
5 (9.4) |
|
|
|
≥ 5 |
90 (32.6) |
87 (39) |
3 (5.7) |
|
|
|
Unknown |
41 (14.8) |
34 (15.2) |
7 (13.2) |
|
|
|
Oncologic treatment |
|
|
|
|
|
|
ART |
51 (18.5) |
51 (22.9) |
0 |
<0.001 b |
|
|
Chemotherapy |
124 (44.9) |
110 (49.3) |
14 (26.4) |
|
|
|
Radiotherapy |
4 (1.4) |
1 (0.4) |
3 (5.7) |
|
|
|
Surgery |
14 (5.1) |
7 (3.2) |
7 (13.2) |
|
|
|
QT + RT |
14 (5.1) |
9 (4.1) |
5 (9.4) |
|
|
|
QT + QX |
3 (1.1) |
1 (0.4) |
2 (3.8) |
|
|
|
RT + QX |
2 (0.7) |
1 (0.4) |
1 (1.9) |
|
|
|
Unknown |
64 (23.2) |
43 (19.3) |
21 (39.6) |
|
|
ADN: AIDS-defining neoplasms; NADN: non-AIDS-defining neoplasms; IQR: interquartile range; MSM: men who have sex with men; ART: antiretroviral therapy; QT: chemotherapy; RT: radiotherapy; QX: surgery.
a Mann-Whitney U test, b Chi-square test.
Despite the missing data regarding oncologic treatment, 51.1% received chemotherapy in association with other drugs or as the only treatment. For patients with non-disseminated Kaposi’s sarcoma, the initiation of ART as oncologic treatment was immediate upon cancer diagnosis. For the remaining patients with other types of cancer, the median time from cancer diagnosis to oncologic treatment excluding ART was 29 days (IQR, 10-72). Table 3 describes the seven patients who developed a second cancer.
Table 3. Characteristics of patients who developed a second type of cancer diagnosed at Cayetano Heredia Hospital between 2000-2018 (n = 7).
|
Case |
Sex |
Age at first cancer |
Method of transmission |
First cancer |
Second cancer |
|
1 |
F |
43 |
Heterosexual |
ICC |
Lung cancer |
|
2 |
M |
26 |
MSM |
KS |
NHL |
|
3 |
M |
38 |
Heterosexual |
KS |
NHL |
|
4 |
M |
37 |
MSM |
NHL |
HL |
|
5 |
M |
30 |
MSM |
NHL |
Skin cancer |
|
6 |
M |
44 |
MSM |
NHL |
KS |
|
7 |
F |
34 |
Heterosexual |
KS |
ICC |
F: female; M: male; MSM: men who have sex with men; ICC: invasive cervical cancer; KS: Kaposi’s sarcoma; NHL: non-Hodgkin’s lymphoma; HL: Hodgkin's lymphoma.
At the time of cancer diagnosis, NADN cases had a median age of 42.2 years and for ADN cases the median age was 35.2 years. The female to male distribution was 1:4; however, among NADN it was 2:3. The median time from HIV diagnosis to cancer among AND cases was 5.8 months (IQR: 1.1-38.1), while among NADN cases it was 52.7 months (IQR: 16.8- 111.1), demonstrating that the latter group had a longer history of HIV infection. ADN cases had lower CD4 cell counts (median 87.5 cells/µL, IQR: 37-241) than NADN cases (median 216 cells/µL, IQR: 129-298), and viral load was undetectable in 58.5% (31/53) of NADN compared to 17.5% (39/223) of AND cases. Figure 1 shows how the number of cancer cases has been increasing over the years, with ADN cases predominating over NADN.
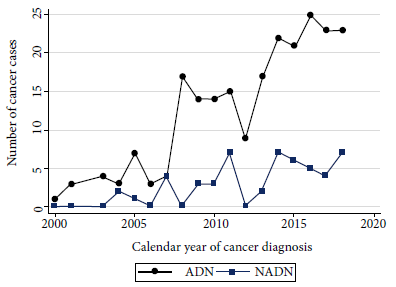
Figure 1. Cases diagnosed with cancer according to their classification into AIDS-defining neoplasms (ADN) and non-AIDS-defining neoplasms (NADN) at Cayetano Heredia Hospital between 2000-2018.
DISCUSSION
This study demonstrates that ADN were the most frequent group of neoplasms among PLHIV, and KS WAS the most common type of cancer. The characteristics of ADN and NADN were opposite in almost all the analyzed variables, thus, NADN were diagnosed at an older age, were mostly heterosexual patients, had a longer HIV history, and at the time of cancer diagnosis, higher CD4 cell count and lower viral load levels.
These findings represent a first insight into cancer in patients with HIV-AIDS, since to date there are no studies that jointly evaluate ADN and NADN. Despite the limitations implied by their descriptive nature, the results did not differ from other studies. Similar results were observed in high- and low-income settings (1,14,15), where AND cases were found to be the predominant group; however, some recent reports from developed countries have shown that the incidence of NADN has increased (6,7). According to previous reports (12,14), KS was the most common neoplasm among ADN, particularly in places where co-infection with HHV-8 is high, such as in Latin America and Africa. Whereas, among NADN, Hodgkin’s lymphoma (HL) was the most frequent, as seen in other countries (16).
Regarding demographic characteristics, in concordance with other studies (17,18), PLHIV were diagnosed with NADN at an older age than ADN; however, it is still a very young age to develop cancer compared to the general population without HIV. Similar to a previous report (19), cases in males were more frequent than in females; however, some studies in Africa have shown that cancer frequencies were comparable or even higher in females because of the higher incidence of HIV among females. Due to the high prevalence of co-infection with HHV-8 among MSM, this population was more likely to belong to the ADN group. On the other hand, although in our series the presence of a second cancer diagnosis was low (seven cases), we noted that most of them were HHV-8 and EBV-related infections.
NADN also had a longer history of HIV infection, higher CD4 cell count, and a higher number of cases in viral suppression than those with ADN. Meijide H. et al. (18), found that NADN cases had an average time from HIV diagnosis to cancer of 8 years compared to ADN cases, who had one year; likewise, about 70% of NADN had CD4 cell counts greater than 200 cells/µL, and almost 60% had an undetectable viral load. A second study conducted in Mexico City by Cornejo-Juarez et al. (20) reported that almost 60% of their cases had CD4 cell counts greater than 200 cells/µL and an undetectable viral load. These findings could mean that either immunosuppression or viral suppression does not play an important role in the development of non-AIDS-related cancers. It is important to develop conduct cohort studies with larger sample size and, therefore, greater power; in addition to including confounding variables so that by means of regression models we can establish whether there is an association between HIV diagnosis and the development of NADN.
The main limitation of this study was based on information bias, since we used records from multiple databases belonging to the HIV cohort, which had information from different sources, such as medical records, discharge reports and histological reports. Likewise, it was not possible to collect data on some clinical variables that would have enriched the study analysis, such as, for example, traditional risk factors for cancer (alcoholism and smoking), co-infections with oncogenic viruses, cancer stage and remission after treatment. Selection bias is also present, since all HIV patients who developed some type of cancer were selected from a single hospital center.
In conclusion, our findings are similar to other case series worldwide, where NADN were the most frequent type of cancer. NADN cases presented older age, longer history of HIV infection, higher CD4 cell counts, and most were in viral suppression.
REFERENCES
1. Hessol NA, Whittemore H, Vittinghoff E, Hsu LC, Ma D, Scheer S, et al. Incidence of first and second primary cancers diagnosed among people with HIV, 1985-2013: a population-based, registry linkage study. Lancet HIV. 2018;5(11):e647-e655. doi: 10.1016/S2352-3018(18)30179-6.
2. Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495-e504. doi: 10.1016/S2352-3018(17)30125-X.
3. Cobucci RN, Lima PH, de Souza PC, Costa VV, Cornetta Mda C, Fernandes JV, et al. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: a systematic review. J Infect Public Health. 2015;8(1):1-10. doi: 10.1016/j.jiph.2014.08.003.
4. Yarchoan R, Uldrick TS. HIV-Associated Cancers and Related Diseases. N Engl J Med 2018; 378:1029-41. doi: 10.1056/NEJMra1615896.
5. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6–11. doi: 10.1097/COH.0000000000000327.
6. Chiu CG, Smith D, Salters KA, Zhang W, Kanters S, Milan D, et al. Overview of cancer incidence and mortality among people living with HIV/AIDS in British Columbia, Canada: Implications for HAART use and NADM development. BMC Cancer. 2017;17(1):270. doi: 10.1186/s12885-017-3229-1.
7. Brickman C, Palefsky JM. Cancer in the HIV-Infected Host: Epidemiology and Pathogenesis in the Antiretroviral Era. Curr HIV/AIDS Rep. 2015;12(4):388-96. doi: 10.1007/s11904-015-0283-7.
8. Mohanna S, Echaíz J, Ferrufino JC, Bravo F, Gotuzzo E. Perfil clínico y epidemiológico del sarcoma de Kaposi clásico y epidémico: estudio retrospectivo en el Hospital Nacional Cayetano Heredia. Folia dermatol Peru. 2006; 17 (3):111-117.
9. Cuellar LE, Anampa-Guzmán A, Holguín AM, Velarde J, Portillo-Alvarez D, Zuñiga-Ninquispe MA, et al. Prognostic factors in HIV-positive patients with non-Hodgkin lymphoma: a Peruvian experience. Infect Agents Cancer. 2018;13:27. doi: 10.1186/s13027-018-0200-y.
10. Castel AD, Young H, Akiwumi AM, Vargas A, Rogers K, West T, et al. Trends in cancer diagnoses and survival among persons with AIDS in a high HIV prevalence urban area. AIDS Care. 2015;27(7):860-9. doi: 10.1 080/09540121.2015.1011074.
11. Hleyhel M, Belot A, Bouvier AM, Tattevin P, Pacanowski J, Genet P, et al. Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis. 2013;57(11):1638-47. doi: 10.1093/cid/cit497.
12. Bohlius J, Valeri F, Maskew M, Prozesky H, Garone D, Sengayi M, et al. Kaposi’s Sarcoma in HIV-infected patients in South Africa: Multicohort study in the antiretroviral therapy era. Int J Cancer. 2014;135(11):2644-2652. doi: 10.1002/ijc.28894.
13. Fink VI, Jenkins CA, Castilho JL, Person AK, Shepherd BE, Grinsztejn B, et al. Survival after cancer diagnosis in a cohort of HIV-positive individuals in Latin America. Infect Agent Cancer. 2018;13:16. doi: 10.1186/s13027-018-0188-3.
14. Fink VI, Shepherd BE, Cesar C, Krolewiecki A, Wehbe F, Cortés CP, et al. Cancer in HIV-infected persons from the Caribbean, Central and South America. J Acquir Immune Defic Syndr. 2011;56(5):467–473. doi: 10.1097/QAI.0b013e31820bb1c3.
15. Álvarez-Guevara Deisy, Cuervo-Maldonado Sonia, Sánchez Ricardo, Gómez-Rincón Julio, Ramírez Nancy. Prevalence of defining malignancies in adult patients with HIV/AIDS in the National Cancer Institute of Colombia. 2007-2014. Rev Fac Med. 2017; 65(3): 397-402. doi: http://dx.doi.org/10.15446/revfacmed.v65n3.56112.
16. Hleyhel M, Bouvier AM, Belot A, Tattevin P, Pacanowski J, Genet P, et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS. 2014;28(14):2109-18. doi: 10.1097/QAD.0000000000000382.
17. Ramírez-Olivencia G, Valencia-Ortega ME, Martin-Carbonero L, More-no-Celda V, González-Lahoz J. Malignancies in HIV infected patients: study of 139 cases. Med Clin (Barc). 2009;133(19):729-35. doi: 10.1016/j.medcli.2009.03.043.
18. Meijide Héctor, Mena Alvaro, Pernas Berta, Castro Ángeles, López Soledad, Vázquez Pilar, et al. Neoplasias en pacientes con infección por VIH: Estudio descriptivo de 129 casos en el período 1993-2010. Rev Chil Infectol. 2013; 30(2): 156-161. doi: http://dx.doi.org/10.4067/S071610182013000200006.
19. Yang J, Su S, Zhao H, Wang D, Wang J, Zhang F, et al. Prevalence and mortality of cancer among HIV-infected inpatients in Beijing, China. BMC Infect Dis. 2016;16:82. doi: 10.1186/s12879-016-1416-3.
20. Cornejo-Juárez P, Cavildo-Jerónimo D, Volkow-Fernández P. Non-AIDS defining cancer (NADC) among HIV-infected patients at an oncology tertiary-care center in Mexico. AIDS Res Ther. 2018;15(1):16. doi: 10.1186/s12981-018-0202-2.
Funding: Program for Advanced Research Capacities for AIDS in Peru (PARACAS) - 5D43TW009763-05.
Cite as: Mendoza-Mori LM, Valenzuela-Medina JB, Gotuzzo E, Mejía-Cordero FA, González-Lagos EV. [Cancer in people living with HIV-AIDS at a referral hospital in Lima, Peru]. Rev Peru Med Exp Salud Publica. 2021;38(2):278-83. doi: https://doi.org/10.17843/rpmesp.2021.382.6341.
Correspondence: Lucía Maryelena Mendoza Mori; lucia.mendoza.m@upch.pe
Author contributions: EGH, FMC and LMM participated in the conception and design of the article. LMM and JVM carried out data collection, analysis and interpretation. LMM and JVM wrote the article. EGH, FMC and EGL provided the study material, and participated in the critical revision of the content of the manuscript. All authors approved the final version of the article and assume responsibility for its content.
Conflict of interest: The authors declare that they have no conflicts of interest.
Received: 13/08/2020
Approved: 12/03/2021
Online: 04/06/2021